Paper:
Olfactory Cues to Reduce Retrograde Interference During the Simultaneous Learning of Conflicting Motor Tasks
Eiko Matsuda*, Daichi Misawa**, Shiro Yano**, and Toshiyuki Kondo**
*Interfaculty Initiative in Information Studies, The University of Tokyo
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
**Department of Computer and Information Sciences, Graduate School of Engineering, Tokyo University of Agriculture and Technology
2-24-16 Naka-cho, Koganei-shi, Tokyo 184-0012, Japan
We investigated the ability of humans to adapt to a novel environment by kinematic transformation. This adaptation was studied via behavioural experiments using a robotic manipulandum – a system designed to arbitrarily generate virtual force fields against a human hand and subsequently record the hand’s trajectory. By repeating motor tasks, this study’s participants gradually learned to move correctly under a newly experienced force field, such as rotating in a clockwise direction. However, each participant’s motor memory was destroyed if he/she experienced an opposing force field (e.g., in a counterclockwise direction) immediately after learning the initial movement, which is known as retrograde interference. In some previous studies, it has been considered that by presenting sensory cues to highlight the difference in two opposing force fields, participants can learn both force fields independently without interference. In this study, we investigated the functionality of olfactory cues – specifically lemon and lavender odors – in reducing retrograde interference. Forty-five university students participated in an experiment using a robotic manipulandum. Our results have shown that the presence of lemon odor reduces the destruction of motor memory, while that of lavender did not, suggesting that odors can enhance simultaneous motor learning but the effect depends on the type of odor used.
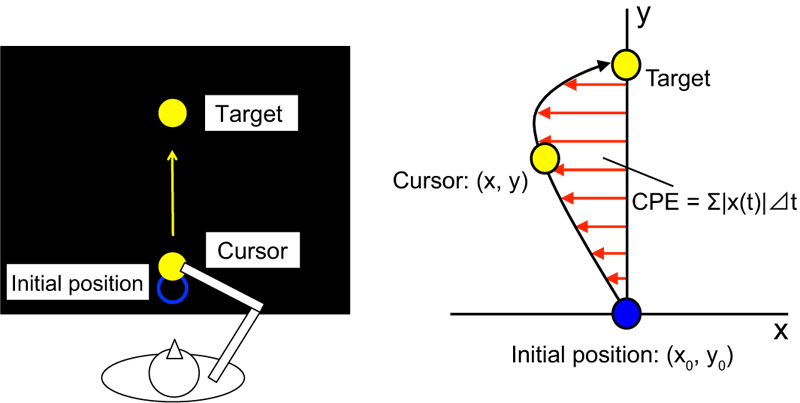
Behavioral experiment using a robotic manipulandum
- [1] T. Brashers-Krug, R. Shadmehr, and E. Bizzi, “Consolidation in human motor memory,” Nature, Vol.382, No.6588, pp. 252-255, 1996.
- [2] R. Shadmehr and T. Brashers-Krug, “Functional stages in the formation of human long-term motor memory,” The J. of Neuroscience, Vol.17, No.1, pp. 409-419, 1997.
- [3] G. Caithness, R. Osu, P. Bays, H. Chase, J. Klassen, M. Kawato, D. M. Wolpert, and J. R. Flanagan, “Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks,” The J. of Neuroscience, Vol.24, Issue 40, pp. 8662-8671, 2004.
- [4] J. W. Krakauer, C. Ghez, and M. F. Ghilardi, “Adaptation to visuomotor transformations: Consolidation, interference, and forgetting,” The J. of Neuroscience, Vol.25, No.2, pp. 473-478, 2005.
- [5] P. M. Bays, J. R. Flanagan, and D. M. Wolpert, “Interference between velocity-dependent and position-dependent force-fields indicates that tasks depending on different kinematic parameters compete for motor working memory,” Experimental Brain Research, Vol.163, pp. 400-405, 2005.
- [6] R. Gupta and J. Ashe, “Lack of adaptation to random conflicting force fields of variable magnitude,” J. of Neurophysiology, Vol.97, Issue 1, pp. 738-745, 2007.
- [7] N. Cothros, J. Wong, and P. L. Gribble, “Distinct haptic cues do not reduce interference when learning to reach in multiple force fields,” PLoS One, Vol.3, No.4, Article No.e1990, 2008.
- [8] T. Sakamoto and T. Kondo, “Visuomotor learning by passive motor experience,” Frontiers in Human Neuroscience, Vol.9, Article No.00279, 2015.
- [9] R. Shadmehr and F. A. Mussa-Ivaldi, “Adaptive representation of dynamics during learning of a motor task,” The J. of Neuroscience, Vol.14, No.5, pp. 3208-3224, 1994.
- [10] J. W. Krakauer, M. F. Ghilardi, and C. Ghez, “Independent learning of internal models for kinematic and dynamic control of reaching,” Nature Neuroscience, Vol.2, No.11, pp. 1026-1031, 1999.
- [11] C. Tong, D. M. Wolpert, and J. R. Flanagan, “Kinematics and dynamics are not represented independently in motor working memory: Evidence from an interference study,” The J. of Neuroscience, Vol.22, Issue 3, pp. 1108-1113, 2002.
- [12] N. Cothros, J. Wong, and P. L. Gribble, “Visual cues signaling object grasp reduce interference in motor learning,” J. of Neurophysiology, Vol.102, Issue 4, pp. 2112-2120, 2009.
- [13] F. Gandolfo, F. A. Mussa-Ivaldi, and E. Bizzi, “Motor learning by field approximation,” Proc. of the National Academy of Sciences of the United States of America, Vol.93, No.9, pp. 3843-3846, 1996.
- [14] Y. Wada, Y. Kawabata, S. Kotosaka, K. Yamamoto, S. Kitazawa, and M. Kawato, “Acquisition and contextual switching of multiple internal models for different viscous force fields,” Neuroscience Research, Vol.46, Issue 3, pp. 319-331, 2003.
- [15] N. Cothros, J. D. Wong, and P. L. Gribble, “Are there distinct neural representations of object and limb dynamics?,” Experimental Brain Research, Vol.173, No.4, pp. 689-697, 2006.
- [16] H. Imamizu, N. Sugimoto, R. Osu, K. Tsutsui, K. Sugiyama, Y. Wada, and M. Kawato, “Explicit contextual information selectively contributes to predictive switching of internal models,” Experimental Brain Research, Vol.181, No.3, pp. 395-408, 2007.
- [17] I. S. Howard, D. M. Wolpert, and D. W. Franklin, “The effect of contextual cues on the encoding of motor memories,” J. of Neurophysiology, Vol.109, No.10, pp. 2632-2644, 2013.
- [18] I. S. Howard and D. W. Franklin, “Neural tuning functions underlie both generalization and interference,” PLoS One, Vol.10, No.6, Article No.e0131268, 2015.
- [19] I. S. Howard, J. N. Ingram, D. W. Franklin, and D. M. Wolpert, “Gone in 0.6 seconds: The encoding of motor memories depends on recent sensorimotor states,” The J. of Neuroscience, Vol.32, No.37, pp. 12756-12768, 2012.
- [20] R. Osu, S. Hirai, T. Yoshioka, and M. Kawato, “Random presentation enables subjects to adapt to two opposing forces on the hand,” Nature Neuroscience, Vol.7, pp. 111-112, 2004.
- [21] U. Castiello, G. M. Zucco, V. Parma, C. Ansuini, and R. Tirindelli, “Cross-modal interactions between olfaction and vision when grasping,” Chemical Senses, Vol.31, Issue 7, pp. 665-671, 2006.
- [22] V. Parma, D. Zanatto, and U. Castiello, “Visuo-olfactory integration during action observation and execution of reach-to-grasp movements,” Integrative Systems, Vol.24, No.14, pp. 768-772, 2013.
- [23] F. Tubaldi, C. Ansuini, M. L. Demattè, R. Tirindelli, and U. Castiello, “Effects of olfactory stimuli on arm-reaching duration,” Chemical Senses, Vol.33, Issue 5, pp. 433-440, 2008.
- [24] F. Tubaldi, C. Ansuini, R. Tirindelli, and U. Castiello, “The grasping side of odours,” PLoS One, Vol.3, No.3, Article No.e1795, 2008.
- [25] F. Tubaldi, C. Ansuini, R. Tirindelli, and U. Castiello, “The effects of task-irrelevant olfactory information on the planning and the execution of reach-to-grasp movements,” Chamosensory Perception, Vol.2, pp. 25-31, 2009.
- [26] J. K. Kiecolt-Glaser, J. E. Graham, W. B. Malarkey, K. Porterf, S. Lemeshow, and R. Glaser, “Olfactory influences on mood and autonomic, endocrine, and immune function,” Psychoneuroendocrinology, Vol.33, No.3, pp. 328-339, 2008.
- [27] G. Buchbauer, L. Jirovetz, W. Jäger, C. Plank, and H. Dietrich, “Fragrance compounds and essential oils with sedative effects upon inhalation,” J. of Pharmaceutical Sciences, Vol.82, No.6, pp. 660-664, 1993.
- [28] K. Keville and M. Green, “Aromatherapy: A Complete Guide to the Healing Art,” Crossing Press, 1995.
- [29] S. Price and L. Price, “Aromatherapy for Health Professionals,” Churchill Livingstone, 1999.
- [30] T. Komori, R. Fujiwara, M. Tanida, and J. Nomura, “Potential antidepressant effects of lemon odor in rats,” European Neuropsychopharmacology, Vol.5, No.4, pp. 477-480, 1995.
- [31] N. Goel, H. Kim, and R. P. Lao, “An olfactory stimulus modifies nighttime sleep in young men and women,” Chronobiology Int., Vol.22, No.5, pp. 889-904, 2005.
- [32] R. C. Oldfield, “The assessment and analysis of handedness: The Edinburgh inventory,” Neurophychologia, Vol.9, Issue 1, pp. 97-113, 1971.
- [33] S. F. Takagi, “A standardized olfactometer in Japan – a review over ten years,” Annals. of the New York Academy of Sciences, Vol.510, No.1, pp. 113-118, 1989.
- [34] C. Sinding, F. Valadier, V. Al-Hassani, G. Feron, A. Tromelin, I. Kontaris, and T. Hummel, “New determinants of olfactory habituation,” Scientific Reports, Vol.7, Article No.41047, 2017.
- [35] J. L. Emken, R. Benitez, A. Sideris, J. E. Bobrow, and D. J. Reinkensmeyer, “Motor adaptation as a greedy optimization of error and effort,” J. of Neurophysiology, Vol.97, No.6, pp. 3997-4006, 2007.
- [36] C.-W. Hao, W.-S. Lai, C.-T. Ho, and L.-Y. Sheen, “Antidepressant-like effect of lemon essential oil is through a modulation in the levels of norepinephrine, dopamine, and serotonin in mice: Use of the tail suspension test,” J. of Functional Foods, Vol.5, Issue 1, pp. 370-379, 2013.
- [37] K. Tully and V. Y. Bolshakov, “Emotional enhancement of memory: how norepinephrine enables synaptic plasticity,” Molecular Brain, Vol.3, Article No.15, 2010.
- [38] M. A. Diego, N. A. Jones, T. Field, M. Hernandez-Reif, S. Schanberg, C. Kuhn, V. McAdam, R. Galamaga, and M. Galamaga, “Aromatherapy positively affects mood, EEG patterns of alertness and math computations,” Int. J. of Neuroscience, Vol.96, Nos.3-4, pp. 217-224, 1998.
- [39] M. Proust, “Du coté de chez Swann,” Gaillimard, 1919 (in French).
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.