Paper:
Novel Method for Analyzing Flexible Locomotion Patterns of Animals by Using Polar Histogram
Keisuke Naniwa*, Yasuhiro Sugimoto**, Koichi Osuka**, and Hitoshi Aonuma*
*Hokkaido University
Kita 12, Nishi 7, Kita-ku, Sapporo, Hokkaido 060-0812, Japan
**Osaka University
2-1 Yamadaoka, Suita, Osaka 565-0871, Japan
In general, legged robots are designed to walk with a fixed rhythmic pattern. However, most animals can adapt their limb movements while walking. It is necessary to understand the mechanism of adaptability during locomotion when designing bio-inspired legged robots. In this paper, we propose an approach to analyze the flexible locomotion pattern of animals using a polar histogram. Field crickets were used to investigate variations in leg movement of insects depending on the environment. Crickets have a tripod gait; however, their leg movement changes depending on the texture of the ground. There was a significant difference between the leg movement when walking and when swimming. Our approach can explain how animals move their legs during locomotion. This study is useful for evaluating the movements of legged robots.
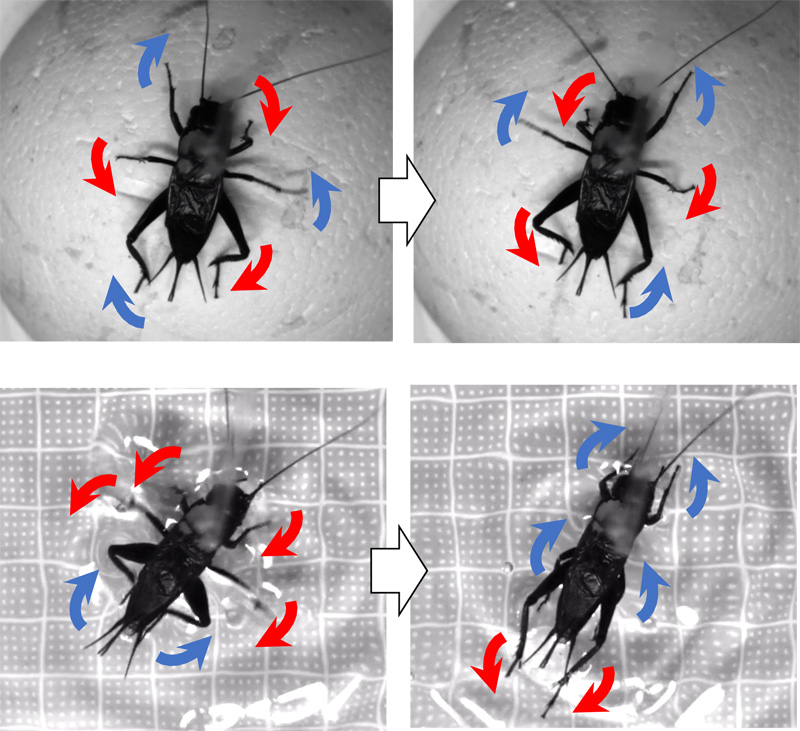
Snapshot of a cricket walking and swimming
- [1] H. Adachi, N. Koyachi, T. Nakamura, and N. Eiji, “Development of quadruped walking robots and their gait study,” J. Robot. Mechatron., Vol.5, No.6, pp. 548-560, 1993.
- [2] P. Arena, L. Fortuna, M. Frasca, and G. Sicurella, “An adaptive, self-organizing dynamical system for hierarchical control of bio-inspired locomotion,” IEEE Trans. on Systems, Man, and Cybernetics, Part B: Cybernetics, Vol.34, No.4, pp. 1823-1837, 2004.
- [3] R. W. Sinnet and A. D. Ames, “Bio-inspired feedback control of three-dimensional humanlike bipedal robots,” J. Robot. Mechatron., Vol.24, No.4, pp. 595-601, 2012.
- [4] D. Owaki, T. Kano, K. Nagasawa, A. Tero, and A. Ishiguro, “Simple robot suggests physical interlimb communication is essential for quadruped walking,” J. of the Royal Society Interface, Vol.10, No.78, 20120669, 2013.
- [5] T. Kinugasa and Y. Sugimoto, “Dynamically and biologically inspired legged locomotion: A review,” J. Robot. Mechatron., Vol.29, No.3, pp. 456-470, 2017.
- [6] K. Osuka, T. Kinugasa, R. Hayashi, K. Yoshida, D. Owaki, and A. Ishiguro, “Centipede type robot i-centipot: From machine to creatures,” J. Robot. Mechatron., Vol.31, No.5. pp. 723-726, 2019.
- [7] K. Kawabata, T. Fujiki, Y. Ikemoto, H. Aonuma, and H. Asama, “A neuromodulation model for adaptive behavior selection by the cricket – nitric oxide (no)/cyclic guanosine monophosphate (cgmp) cascade model –,” J. Robot. Mechatron., Vol.19, No.4, pp. 388-394, 2007.
- [8] T. Matsuura, M. Kanou, and T. Yamaguchi, “Motor program initiation and selection in crickets, with special reference to swimming and flying behavior,” J. of Comparative Physiology – A Sensory, Neural, and Behavioral Physiology, Vol.187, No.12, pp. 987-995, 2002.
- [9] M. Sakura and H. Aonuma, “Aggressive behavior in the antennectomized male cricket Gryllus bimaculatus,” J. of Experimental Biology, Vol.216, No.12, pp. 2221-2228, 2013.
- [10] H. F. Donald and C. R. Taylor, “Gait and the energetics of Locomotion in Horses,” Nature, Vol.292, Issue 5820, pp. 239-240, 1981.
- [11] D. Graham, “A behavioural analysis of the temporal organisation of walking movements in the 1st instar and adult stick insect (Carausius morosus),” J. of Comparative Physiology, Vol.81, No.1, pp. 23-52, 1972.
- [12] C. S. Mendes, I. Bartos, T. Akay, S. Márka, and R. S. Mann, “Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster,” eLife, Vol.2013, No.2, pp. 1-24, 2013.
- [13] A. Wosnitza, T. Bockemüh, M. Dübbert, H. Scholz, and A. Büschges, “Inter-leg coordination in the control of walking speed in Drosophila,” J. of Experimental Biology, Vol.216, No.3, pp. 480-491, 2013.
- [14] C. P. E. Zollikofer, “Stepping Patterns in Ants,” J. of Experimental Biology, Vol.192, pp. 119-127, 1994.
- [15] K. Naniwa, Y. Sugimoto, K. Osuka, and H. Aonuma, “Defecation initiates walking in the cricket Gryllus bimaculatus,” J. of Insect Physiology, Vol.112, pp. 117-122, 2019.
- [16] W. L. Miller and K. A. Sigvardt, “Spectral analysis of oscillatory neural circuits,” J. of Neuroscience Methods, Vol.80, No.2, pp. 113-128, 1998.
- [17] M. Gruhn, G. Von Uckermann, S. Westmark, A. Wosnitza, A. Büschges, and A. Borgmann, “Control of stepping velocity in the stick insect Carausius morosus,” J. of Neurophysiology, Vol.102, No.2, pp. 1180-1192, 2009.
- [18] J. A. Bender, A. J. Pollack, and R. E. Ritzmann, “Neural Activity in the Central Complex of the Insect Brain Is Linked to Locomotor Changes,” Current Biology, Vol.20, No.10, pp. 921-926, 2010.
- [19] R. McN. Alexander, “The gaits of bipedal and quadrupedal animals,” The Int. J. of Robotics Research, Vol.3, No.2, pp. 49-59, 1984.
- [20] M. Hildebrand, “Symmetrical gaits of primates,” American J. of Physical Anthropology, Vol.26, No.2, pp. 119-130, 1967.
- [21] M. Hildebrand, “Symmetrical gaits of horses,” Science, Vol.150, No.3697, pp. 701-708, 1965.
- [22] J. J. Robilliard, T. Pfau, and A. M. Wilson, “Gait characterisation and classification in horses,” J. of Experimental Biology, Vol.210, No.2, pp. 187-197, 2007.
- [23] G. Catavitello, Y. P. Ivanenko, and F. Lacquaniti, “Planar covariation of hindlimb and forelimb elevation angles during terrestrial and aquatic locomotion of dogs,” PLoS ONE, Vol.10, No.7, pp. 1-26, 2015.
- [24] T. Kano, D. Owaki, and A. Ishiguro, “A Simple Measure for Evaluating Gait Patterns during Multi-Legged Locomotion,” SICE J. of Control, Measurement, and System Integration, Vol.7, No.4, pp. 214-218, 2014.
- [25] L. M. Theunissen and V. Dürr, “Insects use two distinct classes of steps during unrestrained locomotion,” PLoS ONE, Vol.8, No.12, pp. 1-18, 2013.
- [26] T. Watanabe, H. Sadamoto, and H. Aonuma, “Molecular basis of the dopaminergic system in the cricket Gryllus bimaculatus,” Invertebrate Neuroscience, Vol.13, No.2, pp. 107-123, 2013.
- [27] E. M. Staudacher, “Sensory responses of descending brain neurons in the walking cricket, Gryllus bimaculatus,” J. of Comparative Physiology – A Sensory, Neural, and Behavioral Physiology, Vol.187, No.1, pp. 1-17, 2001.
- [28] M. Kanou, S. Morita, T. Matsuura, and T. Yamaguchi, “Analysis of Behavioral Selection After Sensory Deprivation of Legs in the Cricket Gryllus bimaculatus,” Zoological Science, Vol.24, No.10, pp. 945-952, 2007.
- [29] W. Nemec, “The shape of the rose,” Sedimentary Geology, Vol.59, Nos.1-2, pp. 149-152, 1988.
- [30] D. J. Best and N. I. Fisher, “The bias of the maximum likelihood estimators of the von mises-fisher concentration parameters,” Communications in Statistics – Simulation and Computation, Vol.10, No.5, pp. 493-502, 1981.
- [31] M. A. Stephens, “Edf statistics for goodness of fit and some comparisons,” J. of the American Statistical Association, Vol.69, No.347, pp. 730-737, 1974.
- [32] N. I. Fisher and A. J. Lee, “Regression Models for an Angular Response,” Biometrics, Vol.48, pp. 665-677, 1993.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.