Paper:
Vision-Based Real-Time Microflow-Rate Control System for Cell Analysis
Tadayoshi Aoyama, Amalka De Zoysa, Qingyi Gu, Takeshi Takaki, and Idaku Ishii
Department of System Cybernetics, Hiroshima University
1-4-1 Kagamiyama, Higashi-Hiroshima, Hiroshima 739-8527, Japan
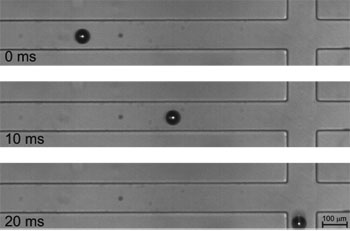
Snapshots of particle sorting experiment using our system
- [1] Y. Chen, T.-H. Wu, Y.-C. Kung, M. A. Teitellbc, and P.-Y. Chiou, “3d pulsed laser-triggered high-speed microfluidic fluorescence-activated cell sorter,” Analyst, Vol.138, pp. 7308-7315, 2013.
- [2] L. Luo and S. Akella, “Optimal scheduling for biochemical analyses on digital microfluidic systems,” IEEE Trans. on Automation Science and Engineering, Vol.8, No.1, pp. 216-227, 2011.
- [3] A. Drochon, “Use of cell transit analyser pulse height to study the deformation of erythrocytes in microchannels,” Medical Engineering and Physics, Vol.27, No.2, pp. 157-165, 2005.
- [4] Y. Katsumoto, K. Tatsumi, T. Doi, and K. Nakabe, “Electrical classification of single red blood cell deformability in high-shear microchannel flows,” Int. J. of Heat and Fluid Flow, Vol.31, pp. 985-995, 2010.
- [5] Q.-Q. Ji, G.-S. Du, M. J. van Uden, Q. Fang, and J. M.J. den Toonder, “Microfluidic cytometer based on dual photodiode detection for cell size and deformability analysis,” Talanta, Vol.111, pp. 178-182, 2013.
- [6] S. C. Hur, N. K. Henderson-MacLennan, E. R. B. McCabec, and D. D. Carlo, “Deformability-based cell classification and enrichment using inertial microfluidics,” Lab on a Chip, Vol.11, pp. 912-920, 2011.
- [7] S. A. Lee, G. Zheng, N. Mukherjeea, and C. Yang, “On-chip continuous monitoring of motile microorganisms on an epetri platform,” Lab on a Chip, Vol.12, pp. 2385-2390, 2012.
- [8] E. Schonbrun, S. S. Gorthi, and D. Schaak, “Microfabricated multiple field of view imaging flow cytometry,” Lab on a Chip, Vol.12, pp. 268-273, 2012.
- [9] S. A. Kobel, O. Burri, A. Griffa, M. Girotra, A. Seitzb, and M. P. Lutolf, “Automated analysis of single stem cells in microfluidic traps,” Lab on a Chip, Vol.12, pp. 2843-2849, 2012.
- [10] I. Moon, B. Javidi, F. Yi, D. Boss, and P. Marquet, “Automated statistical quantification of three-dimensional morphology and mean corpuscular hemoglobin of multiple red blood cells,” Optics Express, Vol.20, No.9, pp. 10295-10309, 2012.
- [11] A. M. Forsyth, “The dynamic behavior of chemically stiffened red blood cells in microchannel flows,” Microvascular Research, Vol.80, No.1, pp. 37-43, 2010.
- [12] D. R. Gossett, H. T. K. Tse, S. A. Lee, Y. Yinge, A. G. Lindgren, O. O. Yang, J. Rao, A. T. Clark, and D. D. Carlo, “Hydrodynamic stretching of single cells for large population mechanical phenotyping,” Proc. of the National Academy of Sciences of the United States of America, Vol.109, No.20, pp. 7630-7635, 2012.
- [13] Q. Gu, T. Aoyama, T. Takaki, and I. Ishii, “Simultaneous vision-based shape and motion analysis of cells fast-flowing in a microchannel,” IEEE Trans. on Automation Science and Engineering, Vol.12, No.1, pp. 204-215, 2015.
- [14] Q. Gu, T. Kawahara, T. Aoyama, T. Takaki, and I. Ishii, “Loc-based high-throughput cell morphology analysis system,” IEEE Trans. on Automation Science and Engineering, Vol.12, No.4, pp. 1346-1356, 2015.
- [15] A. Ichikawa, T. Tanikawa, S. Akagi, and K. Ohba, “Automatic cell cutting by high-precision microfluidic control,” J. of Robotics and Mechatronics, Vol.23, No.1, pp. 13-18, 2011.
- [16] T. Kawahara, M. Sugita, M. Hagiwara, Y. Yamanishi, and F. Arai, “Ultra-high-speed robot hand and eye for investigation of microorganisms in a chip,” Proc. of the 15th Int. Conf. on Miniaturized Systems for Chemistry and Life Sciences, pp. 1427-1429, 2011.
- [17] M. Kobatake, T. Aoyama, T. Takaki, and I. Ishii, “A real-time microscopic piv system using frame straddling high-frame-rate vision,” J. of Robotics and Mechatronics, Vol.25, No.4, pp. 586-595, 2013.
- [18] I. Ishii, T. Tatebe, Q. Gu, Y. Moriue, T. Takaki, and K. Tajima, “2000 fps real-time vision system with high-frame-rate video recording,” Proc. of IEEE Int. Conf. on Robotics and Automation, pp. 1536-1541, 2010.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.