Paper:
Oreochromis niloticus Growth Performance Analysis Using Pixel Transformation and Pattern Recognition
Marife A. Rosales*,†, Argel A. Bandala*, Ryan Rhay P. Vicerra**, Edwin Sybingco*, and Elmer P. Dadios**
*Department of Electronics and Computer Engineering, De La Salle University (DLSU)
2401 Taft Avenue, Malate, Manila 1004, Philippines
**Department of Manufacturing Engineering and Management, De La Salle University (DLSU)
2401 Taft Avenue, Malate, Manila 1004, Philippines
†Corresponding author
To achieve healthy development and optimal growth for harvest in an aquaculture system, correct determination of fish growth stages is very important. The sizes or growth stages of the fish are used by farm managers to regulate stocking densities, optimize daily feeding, and ultimately choose the ideal time for harvesting. This paper presented a vision system-based fish classification using pixel transformation and neural network pattern recognition. Morphometrics parameters are used to facilitate a supervised gathering of datasets. Before feature extraction, the images go through intensity transformation using histogram analysis and Otsu’s thresholding. Using Pearson’s correlation coefficient, the six most important characteristics of the original ten attributes were identified. The developed intelligent model using neural network pattern recognition has an overall training accuracy equal to 90.3%. The validation, test, and overall accuracy are equal to 85.7%, 85.7%, and 88.9%, respectively.
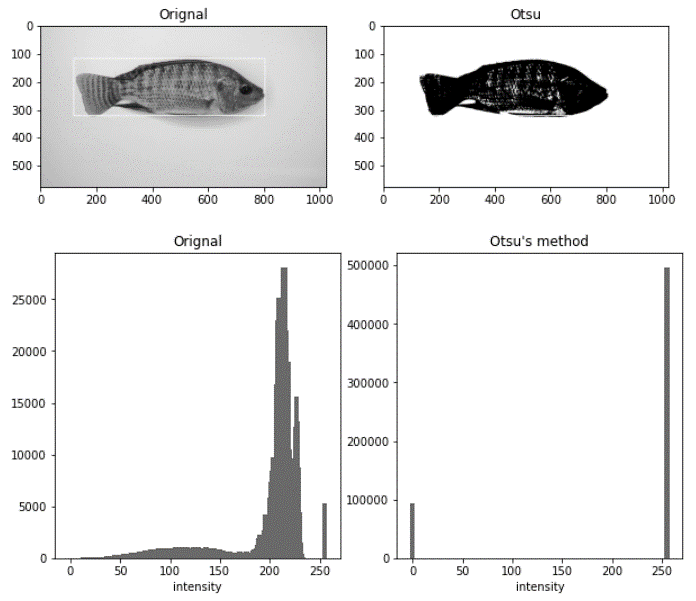
Histogram of original vs. image after Otsu"s thresholding
- [1] C. Wang, Z. Li, T. Wang et al., “Intelligent fish farm – the future of aquaculture,” Aquaculture Int., Vol.29, pp. 2681-2711, 2021.
- [2] “Towards blue transformation,” https://www.fao.org/state-of-fisheries-aquaculture [accessed January 25, 2022]
- [3] R. M. Thomas, A. K. Verma, H. Krishna et al., “Effect of salinity on growth of Nile tilapia (Oreochromis niloticus) and spinach (Spinacia oleracea) in aquaponic system using inland saline groundwater,” Aquac. Res., Vol.52, No.12, pp. 6288-6298, 2021.
- [4] J.-A. V. Magsumbol, V. J. Almero, M. A. Rosales et al., “A Fuzzy Logic Approach for Fish Growth Assessment,” IEEE 11th Int. Conf. on Humanoid, Nanotechnology, Information Technology, Communication and Control, Environment, and Management (HNICEM), doi: 10.1109/HNICEM48295.2019.9072756, 2019.
- [5] M. Saberioon, A. Gholizadeh, P. Cisar et al., “Application of machine vision systems in aquaculture with emphasis on fish: state-of-the-art and key issues,” Rev. Aquac., Vol.9, No.4, pp. 369-387, 2017.
- [6] D. Li, Y. Hao, and Y. Duan, “Nonintrusive methods for biomass estimation in aquaculture with emphasis on fish: a review,” Rev. Aquac., Vol.12, No.3, pp. 1390-1411, 2020.
- [7] H. Li, Y. Chen, W. Li et al., “An adaptive method for fish growth prediction with empirical knowledge extraction,” Biosyst. Eng., Vol.212, pp. 336-346, 2021.
- [8] B. O. Musa, A. Hernández-Flores, O. A. Adeogun et al., “Determination of a predictive growth model for cultivated African catfish Clarias gariepinus (Burchell, 1882),” Aquac. Res., Vol.52, No.9, pp. 4434-4444, 2021.
- [9] C. G. Donohue, G. J. Partridge, and A. M. M. Sequeira, “Bioenergetic growth model for the yellowtail kingfish (Seriola lalandi),” Aquaculture, Vol.531, Article No.735884, 2021.
- [10] M. Jover and V. D. Estruch, “The Quantile Regression Mixed Growth Model Can Help to Improve the Productivity in Gilthead Sea Bream (Sparus aurata) and European Sea Bass (Dicentrarchus labrax) Growing in Marine Farms,” J. Aquac. Mar. Biol., Vol.6, No.4, doi: 10.15406/jamb.2017.06.00161, 2017.
- [11] V. D. Estruch, P. Mayer, B. Roig et al., “Developing a new tool based on a quantile regression mixed-TGC model for optimizing gilthead sea bream (Sparus aurata L) farm management,” Aquac. Res., Vol.48, No.12, pp. 5901-5912, 2017.
- [12] S. A. Flinn and S. R. Midway, “Trends in growth modeling in fisheries science,” Fishes, Vol.6, No.1, doi: 10.3390/fishes6010001, 2021.
- [13] M. A. Rosales, M. G. B. Palconit, V. J. D. Almero et al., “Faster R-CNN based Fish Detector for Smart Aquaculture System,” IEEE 13th Int. Conf. on HNICEM, doi: 10.1109/HNICEM54116.2021.9732042, 2022.
- [14] V. J. D. Almero, R. S. Concepcion II, M. Rosales et al., “An Aquaculture-Based Binary Classifier for Fish Detection Using Multilayer Artificial Neural Network,” IEEE 11th Int. Conf. on HNICEM, doi: 10.1109/HNICEM48295.2019.9072911, 2019.
- [15] M. A. Rosales, J. A. V. Magsumbol, M. G. B. Palconit et al., “Artificial Intelligence: The Technology Adoption and Impact in the Philippines,” IEEE 12th Int. Conf. on HNICEM, doi: 10.1109/HNICEM51456.2020.9400025, 2020.
- [16] M. G. B. Palconit, R. S. Concepcion II, J. D. Alejandrino et al., “Three-dimensional stereo vision tracking of multiple free-swimming fish for low frame rate video,” J. Adv. Comput. Intell. Intell. Inform., Vol.25, No.5, pp. 639-646, 2021.
- [17] C. Shi, Q. Wang, X. He et al., “An automatic method of fish length estimation using underwater stereo system based on LabVIEW,” Comput. Electron. Agric., Vol.173, Article No.105419, 2020.
- [18] A. F. A. Fernandes, E. M. Turra, É. R. d. Alvarenga et al., “Deep Learning image segmentation for extraction of fish body measurements and prediction of body weight and carcass traits in Nile tilapia,” Comput. Electron. Agric., Vol.170, Article No.105274, 2020.
- [19] R. Cheng, C. Zhang, Q. Xu et al., “Underwater fish body length estimation based on binocular image processing,” Information, Vol.11, No.10, Article No.476, 2020.
- [20] A. Álvarez-Ellacuría, M. Palmer, I. A. Catalán et al., “Image-based, unsupervised estimation of fish size from commercial landings using deep learning,” ICES J. Mar. Sci., Vol.77, No.4, pp. 1330-1339, 2020.
- [21] P. Risholm, A. Mohammed, T. Kirkhus et al., “Automatic length estimation of free-swimming fish using an underwater 3D range-gated camera,” Aquac. Eng., Vol.97, Article No.102227, 2022.
- [22] M. G. B. Palconit, V. J. D. Almero, M. A. Rosales et al., “Towards Tracking: Investigation of Genetic Algorithm and LSTM as Fish Trajectory Predictors in Turbid Water,” IEEE Region 10 Conf. (TENCON), pp. 744-749, 2020.
- [23] “Meristics and Morphometrics,” https://fishionary.fisheries.org/meristics_morphometrics/ [accessed July 4, 2022]
- [24] “How to Measure a Fish – Koaw Nature,” https://www.koaw.org/measuring-fishes [accessed February 14, 2022]
- [25] K.-M. Liu, C.-B. Wu, S.-J. Joung et al., “Multi-Model Approach on Growth Estimation and Association with Life History Trait for Elasmobranchs,” Front. Mar. Sci., Vol.8, doi: 10.3389/fmars.2021.591692, 2021.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.