Paper:
Development of Cell Micropatterning Technique Using Laser Processing of Alginate Gel
Haruhiko Takemoto*1, Keito Sonoda*1, Kanae Ike*2, Yoichi Saito*3
 , Yoshitaka Nakanishi*3,*4
, Yoshitaka Nakanishi*3,*4
 , and Yuta Nakashima*3,*4,*5,*6,†
, and Yuta Nakashima*3,*4,*5,*6,†

*1Graduate School of Science and Technology, Kumamoto University
2-39-1 Kurokami, Chuo-ku, Kumamoto, Kumamoto 806-8555, Japan
*2Faculty of Engineering, Kumamoto University
2-39-1 Kurokami, Chuo-ku, Kumamoto, Kumamoto 806-8555, Japan
*3Faculty of Advanced Science and Technology, Kumamoto University
2-39-1 Kurokami, Chuo-ku, Kumamoto, Kumamoto 806-8555, Japan
*4Institute of Industrial Nanomaterials, Kumamoto University
2-39-1 Kurokami, Chuo-ku, Kumamoto, Kumamoto 806-8555, Japan
*5International Research Organization for Advanced Science and Technology, Kumamoto University
2-39-1 Kurokami, Chuo-ku, Kumamoto, Kumamoto 806-8555, Japan
*6Fusion Oriented Research for Disruptive Science and Technology, Japan Science and Technology Agency
5-3 Yonbancho, Chiyoda-ku, Tokyo 102-8666, Japan
†Corresponding author
Tissue formation from heterogeneous cell types, similar to those in vivo, is an important technique for development of new drugs and formation of artificial organs. In vivo tissues are complex arrangements of heterogeneous cells that interact with each other. To create such tissues in vitro, it is essential to develop a technique that arranges heterogeneous cells in an arbitrary configuration. Currently, we are developing a new gel patterning technique to create effective cell micropatterns by using photolithography and alginate gel, which inhibits cellular adhesion. In this study, we considered that a more flexible gel patterning technique was required for creating order-made formations of complex tissues. We created gel patterns by removing the alginate gel using laser processing, and cells were cultured on the formed patterns. Complex heterogeneous cell patterns were achieved by adjusting various technical parameters such as the laser power, spot diameter, and alginate gel film thickness. Based on our results, we anticipate that our technique will prove useful for the development of regenerative medicine and tissue engineering.
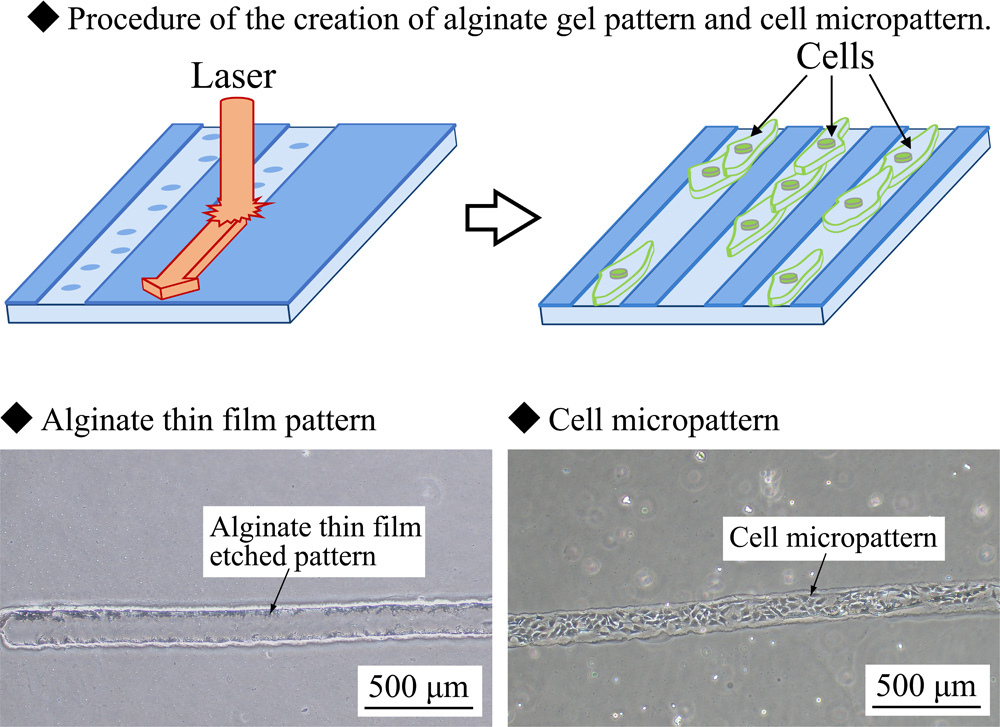
Procedure of gel and cell micropattern
- [1] K. Hoshi et al., “Recent trends in cartilage regenerative medicine and its application to oral and maxillofacial surgery,” Oral Science Int., Vol.10, No.1, pp. 15-19, 2013. https://doi.org/10.1016/S1348-8643(12)00049-3
- [2] H. Sekine and T. Shimizu, “Development of cardiac tissues with the ability for independent cardiac assistance using cell sheet based tissue engineering,” Pediatric Cardiology and Cardiac Surgery, Vol.31, No.3, pp. 88-94, 2015 (in Japanese). https://doi.org/10.9794/jspccs.31.88
- [3] J. Yang et al., “Cell sheet engineering: Recreating tissues without biodegradable scaffolds,” Biomaterials, Vol.26, No.33, pp. 6415-6422, 2005. https://doi.org/10.1016/j.biomaterials.2005.04.061
- [4] T. Shimoto et al., “Study on pipetting motion optimization of automatic spheroid culture system for spheroid formation,” J. Robot. Mechatron., Vol.33, No.1, pp. 78-87, 2021. https://doi.org/10.20965/jrm.2021.p0078
- [5] T. Anada and O. Suzuki, “Size regulation of chondrocyte spheroids using a PDMS-based cell culture chip,” J. Robot. Mechatron., Vol.25, No.4, pp. 644-649, 2013. https://doi.org/10.20965/jrm.2013.p0644
- [6] K. Uesugi et al., “Measuring mechanical properties of cell sheets by a tensile test using a self-attachable fixture,” J. Robot. Mechatron., Vol.25, No.4, pp. 603-610, 2013. https://doi.org/10.20965/jrm.2013.p0603
- [7] H. Ota and N. Miki, “Parallel formation of three-dimensional spheroid using microrotational flow,” J. Robot. Mechatron., Vol.22, No.5, pp. 587-593, 2010. https://doi.org/10.20965/jrm.2010.p0587
- [8] V. Mironov, T. Boland, T. Trusk, G. Forgacs, and R. R. Markwald, “Organ printing: Computer-aided jet-based 3D tissue engineering,” Trends in Biotechnology, Vol.21, No.4, pp. 157-161, 2003. https://doi.org/10.1016/S0167-7799(03)00033-7
- [9] D. N. Heo, M. Hospodiuk, and I. T. Ozbolat, “Synergistic interplay between human MSCs and HUVECs in 3D spheroids laden in collagen/fibrin hydrogels for bone tissue engineering,” Acta Biomaterialia, Vol.95, pp. 348-356, 2019. https://doi.org/10.1016/j.actbio.2019.02.046
- [10] A. Kaneko, Y. Miyazaki, and T. Goto, “Transfer-print of CNTs and its application to cell scaffold,” Int. J. Automation Technol., Vol.11, No.6, pp. 941-946, 2017. https://doi.org/10.20965/ijat.2017.p0941
- [11] A. Kaneko and I. Takeda, “Textured surface of self-assembled particles as a scaffold for selective cell adhesion and growth,” Int. J. Automation Technol., Vol.10, No.1, pp. 62-68, 2016. https://doi.org/10.20965/ijat.2016.p0062
- [12] T. Yasukawa, M. Suzuki, H. Shiku, and T. Matsue, “Fabrication of line and grid patterns with cells based on negative dielectrophoresis,” J. Robot. Mechatron., Vol.22, No.5, pp. 613-618, 2010. https://doi.org/10.20965/jrm.2010.p0613
- [13] J. Kobayashi et al., “Selective cell adhesion and detachment on antibody-immobilized thermoresponsive surfaces by temperature changes,” J. Robot. Mechatron., Vol.25, No.4, pp. 637-643, 2013. https://doi.org/10.20965/jrm.2013.p0637
- [14] Y. Nakashima, K. Tsusu, K. Minami, and Y. Nakanishi, “Development of a cell culture surface conversion technique using alginate thin film for evaluating effect upon cellular differentiation,” Review of Scientific Instruments, Vol.85, Article No.065004, 2014. https://doi.org/10.1063/1.4884076
- [15] H. Terazono et al., “A non-destructive culturing and cell sorting method for cardiomyocytes and neurons using a double alginate layer,” PLOS ONE, Vol.7, No.8, Article No.e42485, 2012. https://doi.org/10.1371/journal.pone.0042485
- [16] F. Ozawa et al., “Alginate gel microwell arrays using electrodeposition for three-dimensional cell culture,” Lab on a Chip, Vol.13, No.15, pp. 3128-3135, 2013. http://doi.org/10.1039/C3LC50455G
- [17] J. A. Rowley, G. Madlambayan, and D. J. Mooney, “Alginate hydrogels as synthetic extracellular matrix materials,” Biomaterials, Vol.20, No.1, pp. 45-53, 1999. https://doi.org/10.1016/S0142-9612(98)00107-0
- [18] M. Shachar, O. Tsur-Gang, T. Dvir, J. Leor, and S. Cohen, “The effect of immobilized RGD peptide in alginate scaffolds on cardiac tissue engineering,” Acta Biomaterialia, Vol.7, No.1, pp. 152-162, 2011. https://doi.org/10.1016/j.actbio.2010.07.034
- [19] Y. Nakashima, Y. Yamamoto, Y. Hikichi, and Y. Nakanishi, “Creation of cell micropatterns using a newly developed gel micromachining technique,” Biofabrication, Vol.8, No.3, Article No.035006, 2011. https://doi.org/10.1088/1758-5090/8/3/035006
- [20] I. Machida-Sano et al., “Surface characteristics determining the cell compatibility of inoically cross-linked alginate gels,” Biomedical Materials, Vol.9, No.2, Arrticle No.025007, 2014. https://doi.org/10.1088/1748-6041/9/2/025007
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.