Paper:
Difference in the Osteoblastic Calcium Signaling Response Between Compression and Stretching Mechanical Stimuli
Katsuya Sato*, Tasuku Nakahara**
 , and Kazuyuki Minami**
, and Kazuyuki Minami**
*Graduate School of Technology, Industrial and Social Sciences, Tokushima University
2-1 Minamijosanjima, Tokushima 770-8506, Japan
**Graduate School of Science and Technology for Innovation, Yamaguchi University
2-16-1 Tokiwadai, Ube, Yamaguchi 755-8611, Japan
In orthodontics, various forms of mechanical stimulation induce opposing bone metabolism mechanisms. Bone resorption and bone formation occur in areas of compressive and tensile force action, respectively. The mechanism that causes such a difference in bone metabolism is still unclear. In this study, we investigated the difference in the osteoblastic calcium signaling response between compression and stretching mechanical stimuli. We applied two types of mechanical stimuli to osteoblast-like MC3T3-E1 cells: first microneedle direct indentation onto the cell as compression stimuli, and second stretching stimuli by using originally developed cell stretching MEMS device. Cells were treated with thapsigargin and calcium-free medium to investigate the source of the calcium ion. The results demonstrated variations in the osteoblastic calcium signaling response between the compression and stretching stimuli. The magnitude of an increase in the intracellular calcium ion concentration is much higher in the compression stimuli-applied cell group. Treatment of calcium-free medium nearly suppressed the calcium signaling response to both types of mechanical stimulation. Thapsigargin treatment induced an increase in the magnitude of calcium signaling response to the compression stimuli, while suppressed the slow and sustained increase in the calcium ion concentration in the stretching stimuli-applied cell group. These findings demonstrate the difference in the characteristics of osteoblastic calcium signaling response between compression and stretching mechanical stimuli.
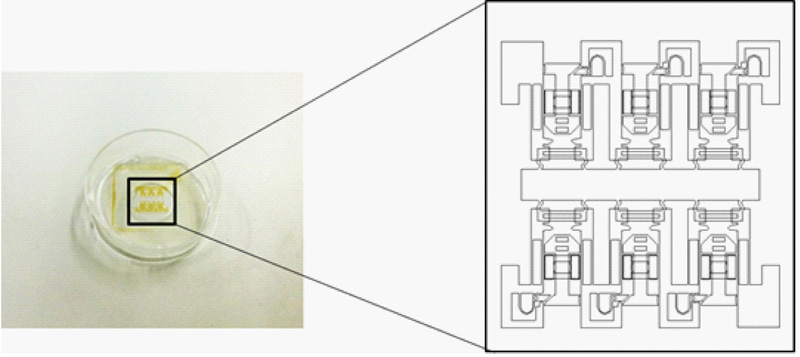
Originally developed cell stretch MEMS device
- [1] C. Verna, M. Dalstra, and B. Melsen, “The rate and the type of orthodontic tooth movement is influenced by bone turnover in a rat model,” European J. Orthodontics, Vol.22, Issue 4, pp. 343-352, 2000. https://doi.org/10.1093/ejo/22.4.343
- [2] A. V. Schepdael, J. V. Sloten, and L. Geris, “A mechanobiological model of orthodontic tooth movement,” Biomech. Modeling in Mechanobiol., Vol.12, No.2, pp. 249-265, 2013. https://doi.org/10.1007/s10237-012-0396-5
- [3] L. M. Godin, S. Suzuki, C. R. Jacobs, H. J. Donahue, and S. W. Donahue, “Mechanically induced intracellular calcium waves in osteoblasts demonstrate calcium fingerprints in bone cell mechanotransduction,” Biomech. Modeling in Mechanobiol., Vol.6, No.6, pp. 391-398, 2007. https://doi.org/10.1007/s10237-006-0059-5
- [4] S. W. Donahue, C. R. Jacobs, and H. J. Donahue, “Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent,” Am. J. Physiol. Cell Phisiol., Vol.281, No.5, pp. C1635-C1641, 2001. https://doi.org/10.1152/ajpcell.2001.281.5.C1635
- [5] M. Zayzafoon, “Calcium/calmodulin signaling controls osteoblast growth and differentiation,” J. Cellular Biochem., Vol.97, No.1, pp. 56-70, 2006. https://doi.org/10.1002/jcb.20675
- [6] K. Sato, S. Kamada, and K. Minami, “Development of microstretching device to evaluate cell membrane strain field around sensing point of mechanical stimuli,” Int. J. Mech. Sci., Vol.52, No.2, pp. 251-256, 2010. https://doi.org/10.1016/J.IJMECSCI.2009.09.021
- [7] K. Naruse and M. Sokabe, “Involvement of stretch-activated ion channels in Ca2+ mobilization to mechanical stretch in endothelial cells,” Am. J. Phisiol. Cell Phisiol., Vol.264, No.4, pp. C1037-C1044, 1993. https://doi.org/10.1152/ajpcell.1993.264.4.C1037
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.