Review:
Swarm Behavior of Adult-Born Neurons During Migration in a Non-Permissive Environment
Naoko Kaneko*,†
 and Taisei Ishimaru*,**
and Taisei Ishimaru*,**
*Laboratory of Neuronal Regeneration, Graduate School of Brain Science, Doshisha University
1-3 Tatara Miyakodani, Kyotanabe, Kyoto 610-0394, Japan
†Corresponding author
**Faculty of Life and Medical Sciences, Doshisha University
1-3 Tatara Miyakodani, Kyotanabe, Kyoto 610-0394, Japan
Much attention has been provided to autonomous decentralized systems based on swarm intelligence algorithms in robotics because of their resistance to component failure and ability to adapt to new environments. During development, various types of collectively migrating cells contribute to tissue and organ formation and have provided useful models for studying swarm behaviors. In the adult brain under physiological conditions, collective cell migration is almost exclusively observed in the rostral migratory stream, where adult-born new neurons travel long distances in contiguous chain-like formation. After ischemic stroke, some new neurons migrate toward the lesion site. Studies show that the promotion of migration is critical for efficient neuronal rewiring in the post-stroke brain in rodents. The new neurons traverse to injured tissues that are not conducive to migration by forming small chains, clearing a path through glial cells, and interacting with blood vessels. Although processes involved in migratory behavior, including cytoskeletal dynamics, intercellular adhesion, and chain formation, have been separately investigated, the mechanisms underlying neuronal swarm behavior are unclear. Future studies should help further our understanding of swarm intelligence and advance the development of novel strategies for controlling neuronal migration to promote efficient functional repair and rewiring in various pathological conditions.
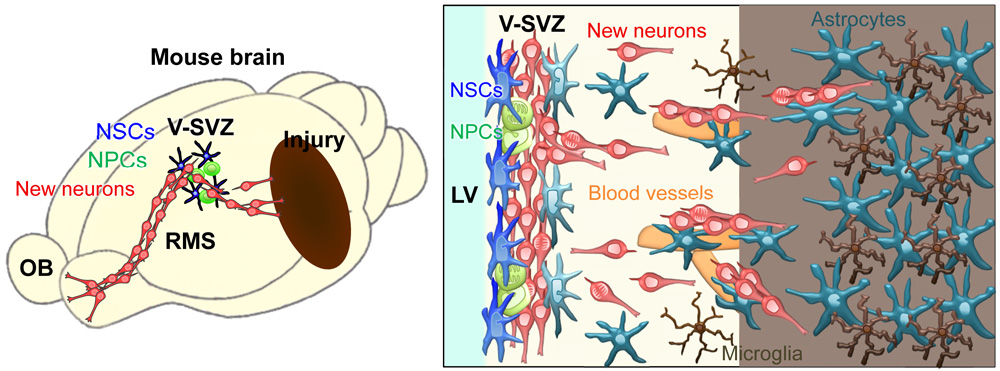
Swarming migration of adult-born neurons
- [1] G. Beni and J. Wang, “Swarm Intelligence in Cellular Robotic Systems,” P. Dario, G. Sandini, and P. Aebischer (Eds.), “Robots and Biological Systems: Towards a New Bionics,” Berlin, Heidelberg: Springer Berlin Heidelberg, pp. 703-712, 1993. https://doi.org/10.1007/978-3-642-58069-7_38
- [2] R. De and D. Chakraborty, “Collective motion: Influence of local behavioural interactions among individuals,” J. Biosci., Vol.47, No.3, Article No.48, 2022. https://doi.org/10.1007/s12038-022-00277-4
- [3] M. Dorigo, M. Birattari, and T. Stutzle, “Ant colony optimization,” IEEE Comput. Intell. Mag., Vol.1, No.4, pp. 28-39, 2006. https://doi.org/10.1109/MCI.2006.329691
- [4] S. Etienne-Manneville, “Neighborly relations during collective migration,” Curr. Opin. Cell Biol., Vol.30, No.1, pp. 51-59, 2014. https://doi.org/10.1016/J.CEB.2014.06.004
- [5] L. J. Schumacher, P. M. Kulesa, R. McLennan, R. E. Baker, and P. K. Maini, “Multidisciplinary approaches to understanding collective cell migration in developmental biology,” Open Biology, Vol.6, No.6, 2016. https://doi.org/10.1098/rsob.160056
- [6] S. J. Streichan, G. Valentin, D. Gilmour, and L. Hufnagel, “Collective cell migration guided by dynamically maintained gradients,” Phys. Biol., Vol.8, No.4, Article No.045004, 2011. https://doi.org/10.1088/1478-3975/8/4/045004
- [7] M. Inaki, S. Vishnu, A. Cliffe, and P. Rørth, “Effective guidance of collective migration based on differences in cell states,” Proc. Natl Acad. Sci. USA, Vol.109, No.6, 2012. https://doi.org/10.1073/pnas.1115260109
- [8] A. Szabó and R. Mayor, “Mechanisms of Neural Crest Migration,” Annu. Rev. Genet., Vol.52, No.1, pp. 43-63, 2018. https://doi.org/10.1146/annurev-genet-120417-031559
- [9] R. McLennan et al., “Multiscale mechanisms of cell migration during development: Theory and experiment,” Development (Cambridge), Vol.139, No.16, pp. 2935-2944, 2012. https://doi.org/10.1242/dev.081471
- [10] R. McLennan et al., “Neural crest migration is driven by a few trailblazer cells with a unique molecular signature narrowly confined to the invasive front,” Development (Cambridge), Vol.142, No.11, pp. 2014-2025, 2015. https://doi.org/10.1242/dev.117507
- [11] L. Qin, D. Yang, W. Yi, H. Cao, and G. Xiao, “Roles of leader and follower cells in collective cell migration,” Molecular Biology of the Cell, Vol.32, No.14, pp. 1267-1272, 2021. https://doi.org/10.1091/mbc.E20-10-0681
- [12] E. Scarpa and R. Mayor, “Collective cell migration in development,” J. of Cell Biology, Vol.212, No.2, pp. 143-155, 2016. https://doi.org/10.1083/jcb.201508047
- [13] F. Doetsch and A. Alvarez-Buylla, “Network of tangential pathways for neuronal migration in adult mammalian brain,” Proc. of the National Academy of Sciences, Vol.93, No.25, pp. 14895-14900, 1996. https://doi.org/10.1073/pnas.93.25.14895
- [14] C. Lois, J.-M. García-Verdugo, and A. Alvarez-Buylla, “Chain Migration of Neuronal Precursors,” Science, Vol.271, No.5251, pp. 978-981, 1996. https://doi.org/10.1126/science.271.5251.978
- [15] D. Lim and A. Alvarez-Buylla, “The Adult Ventricular – Subventricular Zone and Olfactory bulb Neurogenesis,” Cold Spring Harb. Perspect. Biol., Vol.8, No.5, Article No.a018820, 2016.
- [16] K. Obernier and A. Alvarez-Buylla, “Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain,” Development (Cambridge), Vol.146, No.4, 2019. https://doi.org/10.1242/dev.156059
- [17] N. Kaneko, M. Sawada, and K. Sawamoto, “Mechanisms of neuronal migration in the adult brain,” J. Neurochem., Vol.141, No.6, pp. 835-847, 2017. https://doi.org/10.1111/jnc.14002
- [18] B. T. Schaar and S. K. McConnell, “Cytoskeletal coordination during neuronal migration,” Proc. Natl Acad. Sci. USA, Vol.102, No.38, pp. 13652-13657, 2005. https://doi.org/10.1073/pnas.0506008102
- [19] F. Doetsch, J. M. García-Verdugo, and A. Alvarez-Buylla, “Cellular Composition and Three-Dimensional Organization of the Subventricular Germinal Zone in the Adult Mammalian Brain,” The J. of Neuroscience, Vol.17, No.13, pp. 5046-5061, 1997. https://doi.org/10.1523/JNEUROSCI.17-13-05046.1997
- [20] C. Nakajima, M. Sawada, and K. Sawamoto, “Postnatal neuronal migration in health and disease,” Curr. Opin. Neurobiol., Vol.66, pp. 1-9, 2021. https://doi.org/10.1016/j.conb.2020.06.001
- [21] A. Cebrian-Silla et al., “Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenic lineages,” Elife, Vol.10, pp. 1-34, 2021. https://doi.org/10.7554/eLife.67436
- [22] B. Tepe et al., “Single-Cell RNA-Seq of Mouse Olfactory Bulb Reveals Cellular Heterogeneity and Activity-Dependent Molecular Census of Adult-Born Neurons,” Cell Rep., Vol.25, No.10, pp. 2689-2703.e3, 2018. https://doi.org/10.1016/j.celrep.2018.11.034
- [23] N. Kaneko et al., “New neurons clear the path of astrocytic processes for their rapid migration in the adult brain,” Neuron, Vol.67, No.2, pp. 213-223, 2010. https://doi.org/10.1016/j.neuron.2010.06.018
- [24] P. Peretto, C. Giachino, P. Aimar, A. Fasolo, and L. Bonfanti, “Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain,” J. of Comparative Neurology, Vol.487, No.4, pp. 407-427, 2005. https://doi.org/10.1002/cne.20576
- [25] M. F. Paredes et al., “Extensive migration of young neurons into the infant human frontal lobe,” Science, Vol.354, No.6308, Article No.aaf7073, 2016. https://doi.org/10.1126/science.aaf7073
- [26] M. Akter et al., “Dynamic Changes in the Neurogenic Potential in the Ventricular-Subventricular Zone of Common Marmoset during Postnatal Brain Development,” Cereb Cortex, Vol.30, No.7, pp. 4092-4109, 2020. https://doi.org/10.1093/cercor/bhaa031
- [27] H. Hu, “Polysialic acid regulates chain formation by migrating olfactory interneuron precursors,” J. Neurosci. Res., Vol.61, No.5, pp. 480-492, 2000. https://doi.org/10.1002/1097-4547(20000901)61:5<480::AID-JNR2>3.0.CO;2-M
- [28] C. Bressan and A. Saghatelyan, “Intrinsic Mechanisms Regulating Neuronal Migration in the Postnatal Brain,” Front. Cell Neurosci., Vol.14, Article No.620379, 2021. https://doi.org/10.3389/fncel.2020.620379
- [29] N. Kaneko et al., “New neurons use slit-robo signaling to migrate through the glial meshwork and approach a lesion for functional regeneration,” Sci. Adv., Vol.4, No.12, Article No.eaav0618, 2018. https://doi.org/10.1126/sciadv.aav0618
- [30] S. Grade, Y. C. Weng, M. Snapyan, J. Kriz, J. O. Malva, and A. Saghatelyan, “Brain-Derived Neurotrophic Factor Promotes Vasculature-Associated Migration of Neuronal Precursors toward the Ischemic Striatum,” PLoS One, Vol.8, No.1, Article No.e55039, 2013. https://doi.org/10.1371/journal.pone.0055039
- [31] R. L. Zhang et al., “Patterns and dynamics of subventricular zone neuroblast migration in the ischemic striatum of the adult mouse,” J. of Cerebral Blood Flow and Metabolism, Vol.29, No.7, pp. 1240-1250, 2009. https://doi.org/10.1038/jcbfm.2009.55
- [32] T. Kojima et al., “Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum,” Stem Cells, Vol.28, No.3, pp. 545-554, 2010. https://doi.org/10.1002/stem.306
- [33] T. Yamashita et al., “Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum,” J. of Neuroscience, Vol.26, No.24, pp. 6627-6636, 2006. https://doi.org/10.1523/JNEUROSCI.0149-06.2006
- [34] T. Fujioka et al., “β1 Integrin Signaling Promotes Neuronal Migration Along Vascular Scaffolds in the Post-Stroke Brain,” eBioMedicine, Vol.16, pp. 195-203, 2017. https://doi.org/10.1016/j.ebiom.2017.01.005
- [35] H. Wichterle, J. M. García-Verdugo, and A. Alvarez-Buylla, “Direct evidence for homotypic, glia-independent neuronal migration,” Neuron, Vol.18, No.5, pp. 779-791, 1997. https://doi.org/10.1016/S0896-6273(00)80317-7
- [36] T. Hikita, A. Ohno, M. Sawada, H. Ota, and K. Sawamoto, “Rac1-mediated indentation of resting neurons promotes the chain migration of new neurons in the rostral migratory stream of post-natal mouse brain,” J. Neurochem., Vol.128, No.6, pp. 790-797, 2014. https://doi.org/10.1111/jnc.12518
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.