Paper:
Development of a Force Sensor for a Neuroendovascular Intervention Support Robot System
Hiroki Tadauchi*1, Yoshitaka Nagano*2, Shigeru Miyachi*3,*4, Reo Kawaguchi*3, Tomotaka Ohshima*4, and Naoki Matsuo*3
*1Graduate School of Engineering, Aichi University of Technology
50-2 Manori, Nishihasama-cho, Gamagori, Aichi 443-0047, Japan
*2Faculty of Engineering, Aichi University of Technology
50-2 Manori, Nishihasama-cho, Gamagori, Aichi 443-0047, Japan
*3Department of Neurological Surgery, Aichi Medical University
1-1 Yazakokarimata, Nagakute, Aichi 480-1195, Japan
*4Neuroendovascular Therapy Center, Aichi Medical University
1-1 Yazakokarimata, Nagakute, Aichi 480-1195, Japan
Neuroendovascular catheterization using fluoroscopy poses the problem to operators and staffs of cumulative radiation exposure. To solve this problem, we are developing a remote-controlled master-slave robot. Because a wire-like elongated treatment device is inserted into a blood vessel using a catheter, the robot requires a sensor to detect the insertion force of the wire. The proposed sensor is integrated into a robot installed in an X-ray fluoroscopy room that is remotely controlled from another room. The features of this sensor include measurement of the insertion force with sufficient accuracy, simple wire attachment, and an inexpensive disposable sensor head, rendering it very suitable for practical application. In this paper, we report on these features, as well as the results of a practical test of the sensor using a cerebrovascular model.
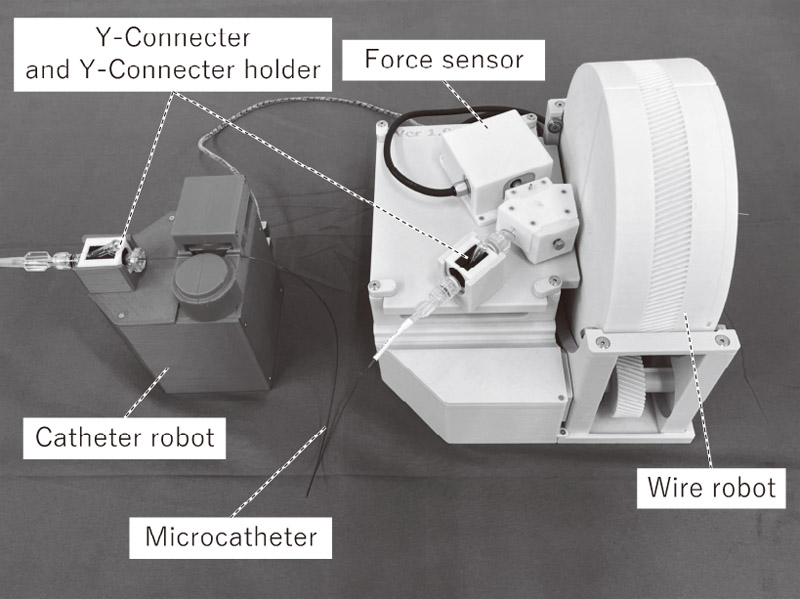
Neuroendovascular intervention support robot
- [1] F. Arai, M. Tanimoto, T. Fukuda, K. Shimojima, H. Matsuura, and M. Negoro, “Multimedia tele-surgery using high speed optical fiber network and its application to intravascular neurosurgery – system configuration and computer networked robotic implementation,” Proc. of IEEE Int. Conf. on Robotics and Automation, Minneapolis, MN, USA, Vol.1, pp. 878-883, doi: 10.1109/ROBOT.1996.503883, 1996.
- [2] M. Tanimoto, F. Arai, T. Fukuda, K. Itoigawa, M. Hashimoto, I. Takahashi, and M. Negoro, “Telesurgery System for Intravascular Neurosurgery,” S. L. Delp, A. M. DiGoia, and B. Jaramaz (Eds.), “Medical Image Computing and Computer-Assisted Intervention – MICCAI 2000,” Berlin, Heidelberg: Springer Berlin Heidelberg, Vol.1935, pp. 29-39, doi: 10.1007/978-3-540-40899-4_4, 2000.
- [3] J. Harrison, L. Ang, J. Naghi, O. Behnamfar, A. Pourdjabbar, M. P. Patel, R. R. Reeves, and E. Mahmud, “Robotically-assisted percutaneous coronary intervention: Reasons for partial manual assistance or manual conversion,” Cardiovascular Revascularization Medicine, Vol.19, No.5, pp. 526-531, doi: 10.1016/j.carrev.2017.11.003, 2018.
- [4] G. Weisz, D. C. Metzger, R. P. Caputo, J. A. Delgado, J. J. Marshall, G. W. Vetrovec, M. Reisman, R. Waksman, J. F. Granada, V. Novack, J. W. Moses, and J. P. Carrozza, “Safety and Feasibility of Robotic Percutaneous Coronary Intervention,” J. of the American College of Cardiology, Vol.61, No.15, pp. 1596-1600, doi: 10.1016/j.jacc.2012.12.045, 2013.
- [5] V. M. Pereira, N. M. Cancelliere, P. Nicholson, I. Radovanovic, K. E. Drake, J.-M. Sungur, T. Krings, and A. Turk, “First-in-human, robotic-assisted neuroendovascular intervention,” J. of NeuroInterventional Surgery, Vol.12, No.4, pp. 338-340, doi: 10.1136/neurintsurg-2019-015671.rep, 2020.
- [6] R. G. Nogueira, R. Sachdeva, A. R. Al-Bayati, M. H. Mohammaden, M. R. Frankel, and D. C. Haussen, “Robotic assisted carotid artery stenting for the treatment of symptomatic carotid disease: technical feasibility and preliminary results,” J. of NeuroInterventional Surgery, Vol.12, No.4, pp. 341-344, doi: 10.1136/neurintsurg-2019-015754, 2020.
- [7] K. C. Sajja, A. Sweid, F. Al Saiegh, N. Chalouhi, M. B. Avery, R. F. Schmidt, S. I. Tjoumakaris, M. R. Gooch, N. Herial, R. Abbas, H. Zarzour, V. Romo, R. Rosenwasser, and P. Jabbour, “Endovascular robotic: feasibility and proof of principle for diagnostic cerebral angiography and carotid artery stenting,” J. of NeuroInterventional Surgery, Vol.12, No.4, pp. 345-349, doi: 10.1136/neurintsurg-2019-015763, 2020.
- [8] K. Haraguchi, S. Miyachi, N. Matsubara, Y. Nagano, H. Yamada, N. Marui, A. Sano, H. Fujimoto, T. Izumi, T. Yamanouchi, T. Asai, and T. Wakabayashi, “A mechanical coil insertion system for endovascular coil embolization of intracranial aneurysms,” Interventional Neuroradiology: J. of Peritherapeutic Neuroradiology, Surgical Procedures and Related Neurosciences, Vol.19, No.2, pp. 159-166, doi: 10.1177/159101991301900203, 2013.
- [9] N. Matsubara, S. Miyachi, T. Izumi, H. Yamada, N. Marui, K. Ota, H. Tajima, K. Shintai, M. Ito, T. Imai, M. Nishihori, and T. Wakabayashi, “Clinical Application of Insertion Force Sensor System for Coil Embolization of Intracranial Aneurysms,” World Neurosurgery, Vol.105, pp. 857-863, doi: 10.1016/j.wneu.2017.06.092, 2017.
- [10] K. Totsu, Y. Haga, and M. Esashi, “Ultra-miniature fiber-optic pressure sensor using white light interferometry,” J. of Micromechanics and Microengineering, Vol.15, No.1, pp. 71-75, doi: 10.1088/0960-1317/15/1/011, 2005.
- [11] S. Tamaki, T. Matsunaga, H. Mushiake, Y. Furusawa, T. Kuki, and Y. Haga, “Development And Evaluation of Tube-Shaped Neural Probe with Working Channel,” The 51st Annual Conf. of Japanese Society for Medical and Biological Engineering, pp. 224-225, 2012.
- [12] M. Tanimoto, F. Arai, T. Fukuda, and M. Negoro, “Force display method to improve safety in teleoperation system for intravascular neurosurgery,” Proc. 1999 IEEE Int. Conf. on Robotics and Automation (Cat. No.99CH36288C), Detroit, MI, USA, Vol.3, pp. 1728-1733. doi: 10.1109/ROBOT.1999.770358, 1999.
- [13] J. Guo, S. Guo, N. Xiao, and B. Gao, “Virtual Reality Simulators Based on a Novel Robotic Catheter Operating System for Training in Minimally Invasive Surgery,” J. Robot. Mechatron., Vol.24, No.4, pp. 649-655, doi: 10.20965/jrm.2012.p0649, 2012.
- [14] S. Guo, Y. Wang, N. Xiao, Y. Li, and Y. Jiang, “Study on real-time force feedback for a master-slave interventional surgical robotic system,” Biomedical Microdevices, Vol.20, No.2, Article No.37, doi: 10.1007/s10544-018-0278-4, 2018.
- [15] Y. Nagano, A. Sano, M. Sakaguchi, and H. Fujimoto, “Development of Force Sensor for Extra-fine and Long Objects,” Trans. of the Society of Instrument and Control Engineers, Vol.44, No.3, pp. 278-284, doi: 10.9746/ve.sicetr1965.44.278, 2008 (in Japanese).
- [16] N. Matsubara, S. Miyachi, Y. Nagano, T. Ohshima, O. Hososhima, T. Izumi, A. Tsurumi, T. Wakabayashi, A. Sano, and H. Fujimoto, “Evaluation of the characteristics of various types of coils for the embolization of intracranial aneurysms with an optical pressure sensor system,” Neuroradiology, Vol.53, No.3, pp. 169-175, doi: 10.1007/s00234-010-0722-5, 2011.
- [17] S. Miyachi, Y. Nagano, T. Hironaka, R. Kawaguchi, T. Ohshima, N. Matsuo, R. Maejima, and M. Takayasu, “Novel Operation Support Robot with Sensory-Motor Feedback System for Neuroendovascular Intervention,” World Neurosurgery, Vol.127, pp. e617-e623, doi: 10.1016/j.wneu.2019.03.221, 2019.
- [18] Y. Nagano, H. Akiyama, S. Miyachi, R. Kawaguchi, and N. Matsuo, “Development of a force sensor in remote operation robot for neuroendovascular treatment,” The 28th Annual Congress of Japan Society of Computer Aided Surgery, Vol.4, pp. 251-252, 2019 (in Japanese).
- [19] S. Miyachi, Y. Nagano, R. Kawaguchi, T. Ohshima, and H. Tadauchi, “Remote surgery using a neuroendovascular intervention support robot equipped with a sensing function: Experimental verification,” Asian J. of Neurosurgery, Vol.16, No.2, pp. 363-366, doi: 10.4103/ajns.AJNS_77_21, 2021.
- [20] G. Guglielmi, F. Viñuela, J. Dion, and G. Duckwiler, “Electrothrombosis of saccular aneurysms via endovascular approach: Part 2: Preliminary clinical experience,” J. of Neurosurgery, Vol.75, No.1, pp. 8-14, doi: 10.3171/jns.1991.75.1.0008, 1991.
- [21] T. Ohshima, Y. Nagano, H. Tadauchi, T. Mase, and S. Miyachi, “Novel Concept and Device for Holding the Rotational Hemostasis Valve During Neuroendovascular Procedures,” World Neurosurgery, Vol.132, pp. 99-102, doi: 10.1016/j.wneu.2019.08.171, 2019.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.