Review:
Neurophysiological Perspective on Allostasis and Homeostasis: Dynamic Adaptation in Viable Systems
Hajime Mushiake
Department of System Neuroscience, Graduate School of Medicine, Tohoku University
2-1 Seiryo-machi, Aoba-ku, Sendai, Miyagi 980-8575, Japan
Allostasis is a physiological principle based on a dynamic regulatory system, contrary to homeostasis, in which the goal is to reach a steady state and recover from deviation from a set point in the internal environment. The concept of allostasis has continued to develop with advances in the field of neuroscience. In this short review, the author presents several new findings in neuroscience and extend the concept of allostasis as mutual regulation between cognitive, somatic, and autonomic systems. In this manner, biological systems adapt to external and internal environments by changing themselves.
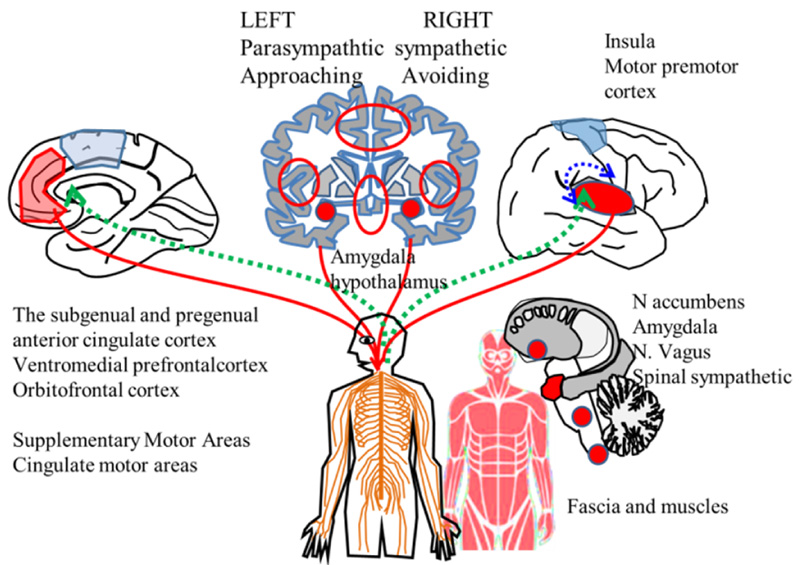
Extended allostatic regulation system
- [1] B. McEwen, “The End of Stress As We Know It,” Joseph Henry Press, 2002.
- [2] J. Schulkin, “Allostasis: a neural behavioral perspective,” Hormones and Behavior, Vol.43, No.1, 2003.
- [3] J. Schulkin and P. Sterling, “Allostasis: a brain-centered, predictive mode of physiological regulation,” Trends Neurosci., Vol.42, No.10, pp. 740-752, 2019.
- [4] P. Sterling and J. Eyer, “Allostasis: a new paradigm to explain arousal pathology,” S. Fisher and J. Reason (Eds.), “Handbook of life stress, cognition and health,” pp. 629-649, John Wiley & Sons, 1988.
- [5] P. Sterling, “Principles of allostasis: optimal design, predictive regulation, pathophysiology and rational therapeutics,” J. Schulkin (Ed.), “Allostasis, Homeostasis, and the Costs of Physiological Adaptation,” Cambridge University Press, 2004.
- [6] K. Friston, “The free-energy principle: a unified brain theory?,” Nat. Rev. Neurosci., Vol.11, pp. 127-138, 2010.
- [7] A. W. Corcoran and J. Hohwy, “Allostasis, interoception, and the free energy principle: Feeling our way forward,” Manos Tsakiris and Helena De Preester (Eds.), “The Interoceptive Mind: From Homeostasis to Awareness,” Oxford University Press, 2018.
- [8] X. Gu and T. H. B. FitzGerald, “Interoceptive inference: Homeostasis and decision-making,” Trends in Cognitive Sciences, Vol.18, No.6, pp. 269-270, 2014.
- [9] A. K. Seth, “Interoceptive inference, emotion, and the embodied self,” Trends in Cognitive Sciences, Vol.17, No.11, pp. 565-573, 2013.
- [10] A. M. Bastos, W. M. Usrey, R. A. Adams, G. R. Mangun, P. Fries, and K. J. Friston, “Canonical microcircuits for predictive coding,” Neuron, Vol.76, No.4, pp. 695-711, 2012.
- [11] G. Buzsáki and A. Draguhn, “Neuronal oscillations in cortical networks,” Science, Vol.304, No.5679, pp. 1926-1929, 2004.
- [12] P. Fries, “Rhythms for Cognition: Communication through Coherence,” Neuron, Vol.88, No.1, pp. 220-235, 2015.
- [13] G. Northoff and H. Mushiake, “Why context matters? Divisive normalization and canonical microcircuits in psychiatric disorders,” Neurosci Res., Vol.156, pp. 130-140, 2020.
- [14] R. Tremblay, S. Lee, and B. Rudy, “GABAergic Interneurons in the Neocortex: From Cellular Properties to Circuits,” Neuron, Vol.91, No.2, pp. 260-292, 2016.
- [15] T. Womelsdorf, T. A. Valiante, N. T. Sahin, K. J. Miller, and P. Tiesinga, “Dynamic circuit motifs underlying rhythmic gain control, gating and integration,” Nat. Neurosci., Vol.17, No.8, pp. 1031-1039, 2014.
- [16] E. T. Rolls, “Cerebral Cortex: Principles of Operation,” Oxford University Press, 2016.
- [17] N. J. Killian and E. A. Buffalo, “Distinct frequencies mark the direction of cortical communication,” Proc. Natl. Acad. Sci., Vol.111, No.40, pp. 14316-14317, 2014.
- [18] T. van Kerkoerle, M. W. Self, B. Dagnino, M.-A. Gariel-Mathis, J. Poort, C. van der Togt, and P. R. Roelfsema, “Alpha and gamma oscillations characterize feedback and feedforward processing in monkey visual cortex,” Proc. Natl. Acad. Sci., Vol.111 No.40, pp. 14332-14341, 2014.
- [19] X.-J. Wang, “Neurophysiological and computational principles of cortical rhythms in cognition,” Physiol Rev., Vol.90, No.3, pp. 1195-1268, 2010.
- [20] R. Hosaka, T. Nakajima, K. Aihara, Y. Yamaguchi, and H. Mushiake, “The Suppression of Beta Oscillations in the Primate Supplementary Motor Complex Reflects a Volatile State During the Updating of Action Sequences,” Cereb Cortex., Vol.26, No.8, pp. 3442-3452, 2016.
- [21] R. Hosaka, H. Watanabe, T. Nakajima, and H. Mushiake, “Theta Dynamics Contribute to Retrieving Motor Plans after Interruptions in the Primate Premotor Area,” Cereb Cortex Commun., Vol.2, No.4, Article No.tgab059, 2021.
- [22] H. Mushiake, M. Inase, and J. Tanji, “Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements,” J. Neurophysiol., Vol.66, No.3, pp. 705-718, 1991.
- [23] J.-S. Brittain, A. Sharott, and P. Brown, “The highs and lows of beta activity in cortico-basal ganglia loops,” Eur. J. Neurosci., Vol.39, No.11, pp. 1951-1959, 2014.
- [24] A. K. Engel and P. Fries, “Beta-band oscillations – signalling the status quo?,” Curr. Opin. Neurobiol., Vol.20, No.2, pp. 156-165, 2010.
- [25] S. Little and P. Brown, “The functional role of beta oscillations in Parkinson’s disease,” Parkinsonism Relat. Disord., Vol.20, Suppl. 1, pp. S44-S48, 2014.
- [26] R. J. Moran, N. Mallet, V. Litvak, R. J. Dolan, P. J. Magill, K. J. Friston, and P. Brown, “Alterations in brain connectivity underlying beta oscillations in Parkinsonism,” PLoS. Comput. Biol., Vol.7, No.8, Article No.e1002124, 2011.
- [27] S. Raghavachari, J. E. Lisman, M. Tully, J. R. Madsen, E. B. Bromfield, and M. J. Kahara, “Theta oscillations in human cortex during a working-memory task: evidence for local generators,” J. Neurophysiol., Vol.95, Issue 3, pp. 1630-1638, 2006.
- [28] E. A. Solomon, J. E. Kragel, M. R. Sperling, A. Sharan, G. Worrell, M. Kucewicz, C. S. Inman, B. Lega, K. A. Davis, J. M. Stein, B. C. Jobst, K. A. Zaghloul, S. A. Sheth, D. S. Rizzuto, and M. J. Kahana, “Widespread theta synchrony and high-frequency desynchronization underlies enhanced cognition,” Nat. Commun., Vol.8, No.1, Article No.1704, 2017.
- [29] B. Voloh, T. A. Valiante, S. Everling, and T. Womelsdorf, “Theta-gamma coordination between anterior cingulate and prefrontal cortex indexes correct attention shifts,” Proc. Natl. Acad. Sci., Vol.112, No.27, pp. 8457-8462, 2015.
- [30] J. F. Cavanagh and M. J. Frank, “Frontal theta as a mechanism for cognitive control,” Trends Cogn. Sci., Vol.18, No.8, pp. 414-421, 2014.
- [31] G. Northoff and S. Tumati, ““Average is good, extremes are bad” – Non-linear inverted U-shaped relationship between neural mechanisms and functionality of mental features,” Neurosci. Biobehav. Rev., Vol.104, pp. 11-25, 2019
- [32] A. D. Craig, “Interoception: the sense of the physiological condition of the body,” Curr. Opin. Neurobiol., Vol.13, No.4, pp. 500-505, 2003.
- [33] I. A. Strigo and A. D. Craig, “Interoception, homeostatic emotions and sympathovagal balance,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., Vol.371, Issue 1708, Article No.20160010, 2016.
- [34] R. P. Dum, D. J. Levinthal, and P. L. Strick, “Motor, cognitive, and affective areas of the cerebral cortex influence the adrenal medulla,” Proc. Natl. Acad. Sci., Vol.113, No.35, pp. 9922-9927, 2016.
- [35] J. Iwata, K. Shima, J. Tanji, and H. Mushiake, “Neurons in the cingulate motor area signal context-based and outcome-based volitional selection of action,” Exp. Brain. Res., Vol.229, No.3, pp. 407-417, 2013.
- [36] K. Shima and J. Tanji, “Role for cingulate motor area cells in voluntary movement selection based on reward,” Science, Vol.282, No.5392, pp. 1335-1338, 1998.
- [37] B. A. Vogt, “Midcingulate cortex: Structure, connections, homologies, functions and diseases,” J. Chem. Neuroanat., Vol.74, pp. 28-46, 2016.
- [38] M. E. Raichle, “The brain’s default mode network,” Annual Review of Neuroscience, Vol.38, pp. 433-447, 2015.
- [39] C. Becchio, A. Cavallo, C. Begliomini, L. Sartori, G. Feltrin, and U. Castiello, “Social grasping: from mirroring to mentalizing,” NeuroImage, Vol.61, No.1, pp. 240-248, 2012.
- [40] U. Frith and C. D. Frith, “Development and neurophysiology of mentalizing,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., Vol.358, No.1431, pp. 459-473, 2003.
- [41] M. V. Lombardo, B. Chakrabarti, E. T. Bullmore, S. J. Wheelwright, S. A. Sadek, J. Suckling, MRC AIMS Consortium, and S. Baron-Cohen, “Shared neural circuits for mentalizing about the self and others,” J. Cogn. Neurosci., Vol.22, No.7, pp. 1623-1635, 2010.
- [42] G. Northoff and J. Panksepp, “The trans-species concept of self and the subcortical-cortical midline system,” Trends Cogn. Sci., Vol.12, No.7, pp. 259-264, 2008.
- [43] R. B. Mars, F.-X. Neubert, M. P. Noonan, J. Sallet, I. Toni, and M. F. S. Rushworth, “On the relationship between the “default mode network” and the “social brain”,” Front. Hum. Neurosci., Vol.6, Article No.189, 2012.
- [44] D. L. Schacter, D. R. Addis, and R. L. Buckner, “Remembering the past to imagine the future: the prospective brain,” Nat. Rev. Neurosci., Vol.8, No.9, pp. 657-661, 2007.
- [45] W. W. Seeley, V. Menon, A. F. Schatzberg, J. Keller, G. H. Glover, H. Kenna, A. L. Reiss, and M. D. Greicius, “Dissociable intrinsic connectivity networks for salience processing and executive control,” J. Neurosci., Vol.27, No.9, pp. 2349-2356, 2007.
- [46] A. Touroutoglou, E. Bliss-Moreau, J. Zhang, D. Mantini, W. Vanduffel, B. C. Dickerson, and L. F. Barrett, “A ventral salience network in the macaque brain,” Neuroimage, Vol.132, pp. 190-197, 2016.
- [47] L. Q. Uddin, “Salience processing and insular cortical function and dysfunction,” Nat. Rev. Neurosci., Vol.16, No.1, pp. 55-61, 2015.
- [48] N. L. Rempel-Clower and H. Barbas, “Topographic organization of connections between the hypothalamus and prefrontal cortex in the rhesus monkey,” J. Comp. Neurol., Vol.398, No.3, pp. 393-419, 1998.
- [49] R. K. Buckner, J. R. Andrews-Hanna, and D. L. Schacter, “The brain’s default network: Anatomy, function, and relevance to disease,” Annals. of the New York Academy of Sciences, Vol.1124, pp. 1-38, 2008.
- [50] M. E. Raichle, “Two views of brain function,” Trends Cogn. Sci., Vol.14, No.4, pp. 180-190, 2012.
- [51] M. E. Raichle, “The restless brain: how intrinsic activity organizes brain function,” Philos. Trans. R. Soc. Lond. B. Biol. Sci., Vol.370, No.1668, Article No.20140172, 2015.
- [52] K. Christoff, Z. C. Irving, K. C. Fox, R. N. Spreng, and J. R. Andrews-Hanna, “Mind-wandering as spontaneous thought: a dynamic framework,” Nat. Rev. Neurosci., Vol.17, No.11, pp. 718-731, 2016.
- [53] M. D. Fox, A. Z. Snyder, J. L. Vincent, M. Corbetta, D. C. van Essen, and M. E. Raichle, “The human brain is intrinsically organized into dynamic, anticorrelated functional networks,” Proc. Natl. Acad. Sci., Vol.102, No.27, pp. 9673-9678, 2005.
- [54] M. Ramot, L. Fisch, M. Harel, S. Kipervasser, F. Andelman, M. Y. Neufeld, U. Kramer, I. Fried, and R. Malach, “A widely distributed spectral signature of task-negative electrocorticography responses revealed during a visuomotor task in the human cortex,” J. Neurosci., Vol.32, No.31, pp. 10458-10469, 2012.
- [55] M. Allen and K. J. Friston, “From cognitivism to autopoiesis: towards a computational framework for the embodied mind,” Synthese., Vol.195, No.6, pp. 2459-2482, 2018.
- [56] G. Pezzulo, F. Rigoli, and K. Friston, “Active Inference, homeostatic regulation and adaptive behavioural control,” Prog. Neurobiol., Vol.134, pp. 17-35, 2015.
- [57] S. Liu, Z.-F. Wang, Y.-S. Su, R. S. Ray, X.-H. Jing, Y.-Q. Wang, and Q. Ma, “Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture,” Neuron, Vol.108, No.3, pp. 436-450.e7, 2020.
- [58] S. Liu, Z. Wang, Y. Su, L. Qi, W. Yang, M. Fu, X. Jing, Y. Wang, and Q. Ma, “A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis,” Nature, Vol.598, pp. 641-645, 2021.
- [59] Y. Shimoda, K. Beppu, Y. Ikoma, Y. M. Morizawa, S. Zuguchi, U. Hino, R. Yano, Y. Sugiura, S. Moritoh, Y. Fukazawa, M. Suematsu, H. Mushiake, N. Nakasato, M. Iwasaki, K. F. Tanaka, T. Tominaga, and K. Matsui, “Optogenetic stimulus-triggered acquisition of seizure resistance,” Neurobiol. Dis., Vol.163, Article No.105602, 2022.
- [60] T. A. Isegar, N. E. R. van Bueren, J. L. Kenemans, R. Gevirtz, and M. Arns, “A frontal-vagal network theory for Major Depressive Disorder: Implications for optimizing neuromodulation techniques,” Brain Stimul., Vol.13, Issue 1, pp. 1-9, 2020.
- [61] L. F. Barrett, “Functionalism cannot save the classical view of emotion,” Soc. Cogn. Affect. Neurosci., Vol.12, No.1, pp. 34-36, 2017.
- [62] L. F. Barrett and W. K. Simmons, “Interoceptive predictions in the brain,” Nature Reviews Neuroscience, Vol.16, pp. 419-429, 2015.
- [63] L. F. Barrett and K. S. Quigley, “Interoception: The Secret Ingredient,” Cerebrum: the Dana forum on brain science, Article No.cer-06-21, 2021.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.