Letter:
Development of Living “Bio-Robots” for Autonomous Actuations
Kazuya Furusawa*, Ryo Teramae**, Hirono Ohashi**, and Masahiro Shimizu**
*Department of Applied Chemistry and Food Science, Fukui University of Technology
3-6-1 Gakuen, Fukui, Fukui 910-8505, Japan
**Department of System Innovation, Osaka University
1-2 Machikaneyama-cho, Toyonaka, Osaka 560-0043, Japan
The implementation of autonomous functions, such as autonomous actuation, self-healing, and learning functions, has been a potent strategy to realize adaptation abilities against changes in environments and sudden incidents. Organic materials, such as living cells and tissues, can be used as robot parts for the implementation of autonomous functions because they can modify biological functions and remodel tissue morphologies in response to the environment. A brain organoid is a cell aggregate formed by recapitulating the development processes of the fetal brain in vitro. Because the brain organoid reproduces complex 3D structures and various cells, it can be used as a living regulator of robots for implementing complex autonomous functions. In contrast, engineered muscle tissues constructed by culturing myoblasts with biomaterials can also be used as a living actuator for robots. Therefore, to implement autonomous functions for robots, we have proposed methods for connecting the brain organoid with engineered muscle tissue and for co-culturing complex in a culture vessel.
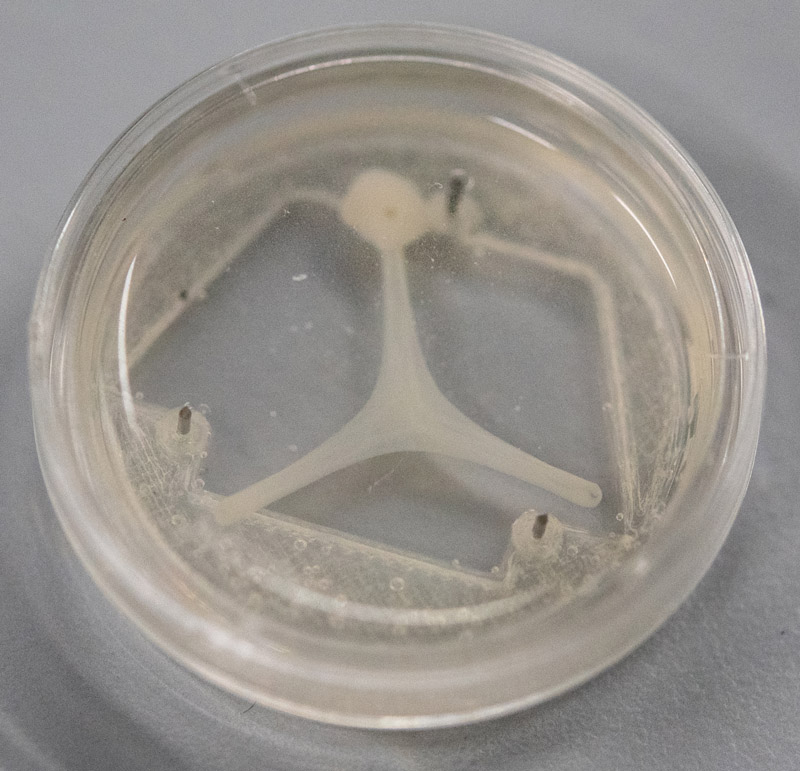
The bio-robot constructed by connecting the brain organoid to 3DCMC
- [1] Y. Morimoto, H. Onoe, and S. Takeuchi, “Biohybrid robot powered by an antagonistic pair of skeletal muscle tissues,” Sci. Robot., Vol.3, Issue 18, eaat4440, doi: 10.1126/scirobotics.aat4440, 2018.
- [2] R. Raman, C. Cvetkovic, S. G. M. Uzel, R. J. Platt, P. Sengupta, R. D. Kamm, and R. Bashir, “Optogenetic skeletal muscle-powered adaptive biological machines,” Proc. of Natl. Acad. Sci. USA, Vol.113, pp. 3497-3502, doi: 10.1073/pnas.1516139113, 2016.
- [3] S. J. Park, M. Gazzola, K. S. Park, S. Park, V. D. Santo, E. L. Blevins, J. U. Lind, P. H. Campell, S. Dauth, A. K. Capulli, F. S. Pasqualini, S. Ahn, A. Cho, H. Yuan, B. M. Maoz, R. Vijaykumar, J.-W. Choi, K. Deisseroth, G. V. Lauder, L. Mahadevan, and K. K. Parker, “Phototactic guidance of a tissue-engineered soft-robotic ray,” Science, Vol.353, Issue 6295, pp. 158-162, doi: 10.1126/science.aaf4292, 2016.
- [4] V. A. Webster-Wood, O. Akkus, U. A. Gurkan, H. J. Chiel, and R. D. Quinn, “Organismal engineering: Toward a robotic taxonomic key for devices using organic materials,” Sci. Robot., Vol.2, eaap9281, doi: 10.1126/scirobotics.aap9281, 2017.
- [5] A. Novellino, P. D’Angelo, L. Cozzi, M. Chiappalone, V. Sanguineti, and S. Martinoia, “Connecting neurons to a mobile robot: An in vitro bidirectional neural interface,” Comput. Intell. Neurosci., Vol.2007, 12725, doi: 10.1155/2007/12725, 2007.
- [6] S. Rajangam, P. H. Tseng, A. Yin, G. Lehew, D. Schwarz, M. A. Lebedev, and M. A. L. Nicolels, “Wireless cortical brain-machine interface for whole-body navigation in primates,” Sci. Rep., Vol.6, 22170, doi: 10.1038/srep22170, 2016.
- [7] M. A. Lancaster, M. Renner, C.-A. Martin, D. Wenzel, L. S. Bicknell, M. E. Hurles, T. Homfray, J. M. Penniger, A. P. Jackson, and J. A. Knoblich, “Cerebral organoids model human brain development and microcephaly,” Nature, Vol.501, pp. 373-379, doi: 10.1038/nature12517, 2013.
- [8] M. A. Lancaster and J. A. Knoblich, “Generation of cerebral organoids from human pluripotent stem cells,” Nat. Protoc., Vol.9, pp. 2329-2340, doi: 10.1038/nprot.2014.158, 2014.
- [9] S. N. Kudoh, C. Hosokawa, A. Kiyohara, T. Taguchi, and I. Hayashi, “Biomodeling system – Interaction between living neuronal networks and the outer world,” J. Robot. Mechatron., Vol.19, pp. 592-600, doi: 10.20965/jrm.2007.p0592, 2007.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.