Paper:
Effect of Inner Diameter and Anticoagulation Coating in a Microneedle on its Blood Suction Performance
Seiji Aoyagi*, Ryosuke Nomura**, Tomokazu Takahashi*, and Masato Suzuki*
*Kansai University
3-3-35 Yamate-cho, Suita, Osaka 564-8680, Japan
**Murata Machinery, Ltd.
136 Takeda-Mukaishiro-cho, Fushimi-ku, Kyoto 612-8686, Japan
As a part of the development of a minimally invasive hollow microneedle designed to mimic a mosquito proboscis, we evaluated the relationship between the needle inner diameter (ID) and blood sucking performance. If the ID is thinned to reduce pain upon piercing skin, blood could clog the tube owing to coagulation, and a sufficient volume of blood might not be obtained. In this study, laser stereo-lithography is used to easily fabricate microtubes of several sizes, at 20–50 μm ID and a fixed length of 500 μm, through which human whole blood is sucked by a vacuum pump. The results indicate that the ID of the tube must be at least 20 μm to prevent hemolysis and at least 50 μm to enable extraction of 200 μL of blood, which is necessary for general blood tests. Moreover, anticoagulant coating applied on the inner wall prevents the clogging of blood and increases the volume of extracted blood.
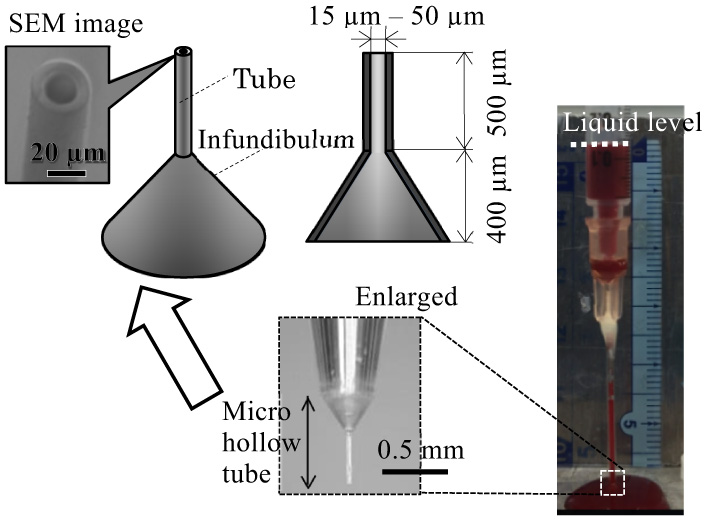
Microtube of 20 μm inner diameter collects 200 μL blood
- [1] K. Oka, S. Aoyagi, Y. Arai, Y. Isono, G. Hashiguchi, and H. Fujita, “Fabrication of a micro needle for a trace blood test,” Sensors and Actuators, Vol.A97-98, pp. 478-485, 2002.
- [2] S. Aoyagi, H. Izumi, and M. Fukuda, “Biodegradable polymer needle with various tip angles and consideration on insertion mechanism of mosquito’s proboscis,” Sensors and Actuators, Vol.A143, No.1, pp. 20-28, 2008.
- [3] H. Izumi, M. Suzuki, S. Aoyagi, and T. Kanzaki, “Realistic imitation of mosquito’s proboscis: electrochemically etched sharp and jagged needles and their cooperative inserting motion,” Sensors and Actuators, Vol.A165, No.1, pp. 115-123, 2011.
- [4] S. Aoyagi, H. Izumi, Y. Isono, M. Fukuda, and H. Ogawa, “Laser fabrication of high aspect ratio thin holes on biodegradable polymer and its application to a microneedle,” Sensors and Actuators, Vol.A139, pp. 293-302, 2007.
- [5] S. Aoyagi, H. Izumi, S. Nakahara, M. Ochi, and H. Ogawa, “Laser microfabrication of long thin holes in biodegradable polymer in vacuum for preventing clogginess and its application to blood collection,” Sensors and Actuators, Vol.A145-146, pp. 464-472, 2008.
- [6] T. Tanaka, T. Takahashi, M. Suzuki, and S. Aoyagi, “Development of minimally invasive microneedle made of tungsten – Sharpening through electrochemical etching and hole processing for drawing up liquid using excimer laser –,” J. Robot. Mechatron., Vol.25, No.4, pp. 755-761, 2013.
- [7] C.-H. Huang, T. Tanaka, Y. Takaoki, H. Izumi, T. Takahashi, M. Suzuki, and S. Aoyagi, “Fabrication of metallic microneedle by electroplating and sharpening of it by electrochemical etching,” IEEJ Trans. Sensors and Micromachines, Vol.131, No.11, pp. 373-380, 2011 (in Japanese).
- [8] M. Suzuki, T. Sawa, T. Takahashi, and S. Aoyagi, “Fabrication of microneedle mimicking mosquito proboscis using nanoscale 3D laser lithography system,” Int. J. Automation Technol., Vol.9, No.6, pp. 655-661, 2015.
- [9] M. Suzuki, T. Takahashi, and S. Aoyagi, “3D laser lithographic fabrication of hollow microneedle mimicking mosquitos and its characterization,” Int. J. Nanotechnology, Vol.15, No.1 pp. 157-173, 2018.
- [10] Y. Hara, M. Yamada, C. Tatsukawa, T. Takahashi, M. Suzuki, and S. Aoyagi, “Fabrication of stainless microneedle with laser cut sharp tip and its characterization of penetration and blood sampling performance,” Int. J. Automation Technol., Vol.10, No.6, pp. 950-957, 2016.
- [11] Y. Hara, M. Yamada, C. Tatsukawa, T. Takahashi, M. Suzuki, and S. Aoyagi, “Laser fabrication of jagged shaped stainless microneedle imitating mosquito’s maxilla,” Int. J. Automation Technol., Vol.10, No.6, pp. 958-964, 2016.
- [12] D. Pickering and J. Marsden, “How to measure blood glucose,” Community Eye Health, Vol 27, No.87, pp. 56-57, 2014.
- [13] L. Heinemann, “Finger pricking and pain: a never ending story,” J. Diabetes Science and Technology, Vol.2, No.5, pp. 919-921, 2008.
- [14] K. Maaden, W. Jiskoot, and J. Bouwstra, “Microneedle technologies for (trans) dermal drug and vaccine delivery,” J. Controlled Release, Vol.161, pp. 645-655, 2012.
- [15] N. Roxhed, T. C. Gasser, P. Griss, G. A. Holzapfel, and G. Stemme, “Penetration-enhanced ultrasharp microneedles and prediction on skin interaction for efficient transdermal drug delivery,” J. MEMS, Vol.16, No.6, pp. 1429-1440, 2007.
- [16] S. Henry, D. V. MecAllister, M. G. Allen, and M. R. Prausnitz, “Micromachined needles for the transdermal delivery of drugs,” Proc. MEMS’98, pp. 494-498, 1998.
- [17] R. S. Woodworth and H. Schlosberg, “Experimental Psychology,” Rinehart and Winston, 1965.
- [18] T. Ikeshouji, “The Interface between Mosquitoes and Humans,” University of Tokyo Press, 1999 (in Japanese).
- [19] A. N. Clement, “The Biology of Mosquitoes,” CABI Publishing, pp. 224-234, 2000.
- [20] J. Wang, S. Ootsuki, T. Takahashi, M. Suzuki, T. Kanzaki, Y. Kawajiri, and T. Oono, “Fabrication of artificial skin with capillary blood vessels and observation of mosquito bite and sucking blood using it,” Proc. Fall Meeting of Japan Soc. Prec. Eng., pp. 113-114, 2014 (in Japanese).
- [21] M. Ooishi, A. Kato, T. Takahashi, M. Suzuki, S. Aoyagi, M. Harada, and M. Ochi, “Observation of inner surface smoothness of mosquito labrum and evaluation of its hydrophilicity,” Proc. Fall Meeting of Japan Soc. Prec. Eng., pp. 761-762, 2015 (in Japanese).
- [22] N. D. M. Begg and A. H. Slocum, “Audible frequency vibration of puncture-access medical devices,” Medical Eng. Physics, Vol.36, No.3, pp. 371-377, 2014.
- [23] S. Aoyagi, Y. Takaoki, H. Takayanagi, C.-H. Huang, T. Tanaka, M. Suzuki, T. Takahashi, T. Kanzaki, and T. Matsumoto, “Equivalent Negative Stiffness Mechanism Using Three Bundled Needles Inspired by Mosquito for Achieving Easy Insertion,” Proc. IEEE/RSJ Int. Conf. Intelligent Robots and Systems (IROS), pp. 2295-2300, 2012.
- [24] X. Q. Kong and C. W. Wu, “Measurement and prediction of insertion force for the mosquito fascicle penetrating into human skin,” J. Bionic Eng., Vol.6, No.2, pp. 143-152, 2009.
- [25] S. J. Moon and S. S. Lee, “Fabrication of microneedle array using inclined LIGA process,” Proc. Transducers’03, pp. 1546-1549, 2003.
- [26] H. Yagyu, S. Hayashi, and O. Tabata, “Fabrication of plastic micro tip array using laser micromachining of nanoparticles dispersed polymer and micromolding,” IEEJ Trans. Sensors and Micromachines, Vol.126, No.1, pp. 7-13, 2006.
- [27] K. Imaeda, K. Bessho, and M. Shikida, “Sharp tip-separable microneedle device for trans-dermal drug delivery systems,” Proc. Transducers 2015, pp. 1715-1718, 2015.
- [28] P. Carraro, G. Servidio, and M. Plebani, “Hemolyzed Specimens: A Reason for Rejection or a Clinical Challenge?,” Clinical Chemistry, Vol.46, No.2, pp. 306-307, 2000.
- [29] N. Watanabe, H. Kataoka, T. Yasuda, and S. Takatani, “Dynamic deformation and recovery response of red blood cells to a cyclically reversing shear flow: effects of frequency of cyclically reversing shear flow and shear stress level,” Biophysical J., Vol.91, pp. 1984-1998, 2006.
- [30] K. Ishihara, T. Ueda, and N. Nakabayashi, “Preparation of phospholipid polymers and their properties as polymer hydrogel membranes,” Polymer J., Vol.22, No.5, pp. 355-360, 1990.
- [31] Y. Iwasaki and K. Ishihara, “Cell membrane-inspired phospholipid polymers for developing medical devices with excellent biointerfaces,” Sci. Technol. Adv. Mater., Vol.13, 064101, 2012.
- [32] C. Cao, A. Kato, T. Takahashi, M. Suzuki, S. Aoyagi, Y. Iwasaki, Y. Ohkubo, and K. Yamamura, “Improvement in hydrophilicity of stainless steel and poly (l-lactic acid) surface coated with MPC copolymer, Proc. JSME Symp. Micro-Nano Science and Technology, 21pm1-E2, 2014 (in Japanese).
- [33] JIS K 0115, “General rules for molecular absorptiometric analysis,” 2004.
- [34] S. Palta, R. Saroa, and A. Palta, “Overview of the coagulation system,” Indian J. Anaesth., Vol.58, No.5, pp. 515-523, 2014.
- [35] E. Ito and T. Okano, “Artificial blood vessels: structure and property of blood contacting surface,” J. Surface Finishing Soc. of Japan, Vol.49, No.7, pp. 45-51, 1998.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.