Letter:
Wearable Biosensor Utilizing Chitosan Biopolymer for Uric Acid Monitoring
Mizuki Sato*1, Tatsuya Kamiyama*2, Kenta Iitani*3
 , Kazuyoshi Yano*4, Kohji Mitsubayashi*3
, Kazuyoshi Yano*4, Kohji Mitsubayashi*3
 , and Takahiro Arakawa*1,*2
, and Takahiro Arakawa*1,*2

*1Graduate School of Engineering, Tokyo University of Technology
1404-1 Katakura, Hachioji, Tokyo 192-0982, Japan
*2Department of Electric and Electronic Engineering, School of Engineering, Tokyo University of Technology
1404-1 Katakura, Hachioji, Tokyo 192-0982, Japan
*3Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University
2-3-10 Kanda-surugadai, Chiyoda-ku, Tokyo 101-0062, Japan
*4Graduate School of Bionics, Computer and Media Sciences, Tokyo University of Technology
1404-1 Katakura, Hachioji, Tokyo 192-0982, Japan
A wearable biosensor was specifically engineered to measure uric acid, a biomarker present at wound sites. This biosensor, fabricated as a disposable and wearable device, was seamlessly integrated onto a polyethylene terephthalate (PET) substrate by utilizing carbon and silver conductive paste as the electrodes. The enzyme uricase was immobilized onto the working electrode by utilizing chitosan, a biocompatible material, to create this biosensor. Notably, the uric acid biosensor fabricated with chitosan showcased exceptional performance metrics, including remarkable output current values and impeccable stability. These findings suggest the prospective utilization of chitosan-based uric acid biosensors for the accurate measurement of uric acid on human skin in future applications.
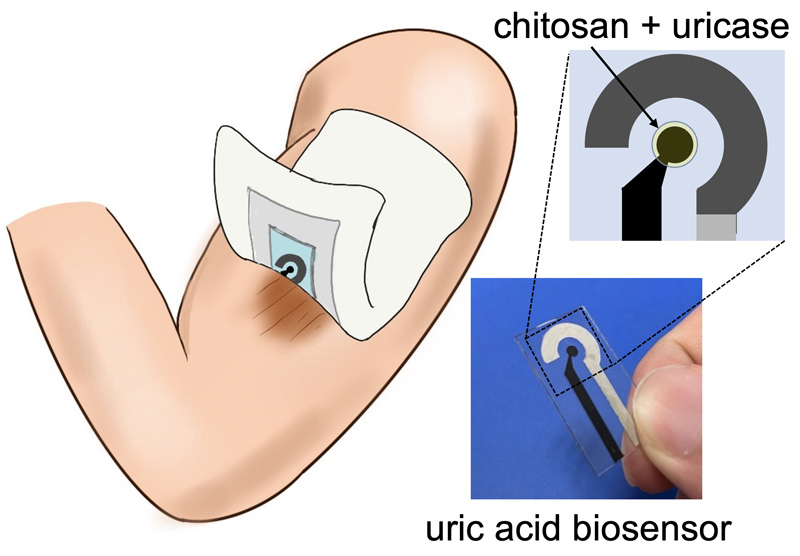
Uric acid biosensor for skin monitoring
- [1] J. R. Windmiller and J. Wang, “Wearable electrochemical sensors and biosensors: A review,” Electroanalysis, Vol.25, No.1, pp. 29-46, 2013. https://doi.org/10.1002/elan.201200349
- [2] J. Kim, A. S. Campbell, B. E.-F. de Ávila, and J. Wang, “Wearable biosensors for healthcare monitoring,” Nat. Biotechnol., Vol.37, No.4, pp. 389-406, 2019. https://doi.org/10.1038/s41587-019-0045-y
- [3] T. Arakawa, D. V. Dao, and K. Mitsubayashi, “Biosensors and chemical sensors for healthcare monitoring: A review,” IEEJ Trans. Electr. Electron. Eng., Vol.17, No.5, pp. 626-636, 2022. https://doi.org/10.1002/tee.23580
- [4] K. Ooe, Y. Hamamoto, T. Kadokawa, and Y. Hirano, “Development of the MOSFET type enzyme biosensor using GOx and ChOx,” J. Robot. Mechatron., Vol.20, No.1, pp. 38-46, 2008. https://doi.org/10.20965/jrm.2008.p0038
- [5] B. K. Ashley, M. S. Brown, Y. Park, S. Kuan, and A. Koh, “Skin-inspired, open mesh electrochemical sensors for lactate and oxygen monitoring,” Biosens. Bioelectron., Vol.132, pp. 343-351, 2019. https://doi.org/10.1016/j.bios.2019.02.041
- [6] H. U. Chung et al., “Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units,” Nat. Med., Vol.26, No.3, pp. 418-429, 2020. https://doi.org/10.1038/s41591-020-0792-9
- [7] T. Arakawa et al., “Real-time monitoring of skin ethanol gas by a high-sensitivity gas phase biosensor (bio-sniffer) for the non-invasive evaluation of volatile blood compounds,” Biosens. Bioelectron., Vol.129, pp. 245-253, 2019. https://doi.org/10.1016/j.bios.2018.09.070
- [8] J. Kim et al., “Simultaneous monitoring of sweat and interstitial fluid using a single wearable biosensor platform,” Adv. Sci., Vol.5, No.10, Article No.1800880, 2018. https://doi.org/10.1002/advs.201800880
- [9] J. Yin et al., “Flexible textile-based sweat sensors for wearable applications,” Biosensors, Vol.13, No.1, Article No.127, 2023. https://doi.org/10.3390/bios13010127
- [10] A. Pal et al., “Early detection and monitoring of chronic wounds using low-cost, omniphobic paper-based smart bandages,” Biosens. Bioelectron., Vol.117, pp. 696-705, 2018. https://doi.org/10.1016/j.bios.2018.06.060
- [11] T. R. Dargaville et al., “Sensors and imaging for wound healing: A review,” Biosens. Bioelectron., Vol.41, pp. 30-42, 2013. https://doi.org/10.1016/j.bios.2012.09.029
- [12] A. Pusta, M. Tertiș, C. Cristea, and S. Mirel, “Wearable sensors for the detection of biomarkers for wound infection,” Biosensors, Vol.12, No.1, Article No.1, 2022. https://doi.org/10.3390/bios12010001
- [13] M. Jose et al., “Stretchable printed device for the simultaneous sensing of temperature and strain validated in a mouse wound healing model,” Sci. Rep., Vol.12, Article No.10138, 2022. https://doi.org/10.1038/s41598-022-13834-6
- [14] S. RoyChoudhury et al., “Continuous monitoring of wound healing using a wearable enzymatic uric acid biosensor,” J. Electrochem. Soc., Vol.165, No.8, pp. B3168-B3175, 2018. https://doi.org/10.1149/2.0231808jes
- [15] P. Kassal et al., “Smart bandage with wireless connectivity for uric acid biosensing as an indicator of wound status,” Electrochem. Commun., Vol.56, pp. 6-10, 2015. https://doi.org/10.1016/j.elecom.2015.03.018
- [16] R. Lungu et al., “Biocompatible chitosan-based hydrogels for bioabsorbable wound dressings,” Gels, Vol.8, No.2, Article No.107, 2022. https://doi.org/10.3390/gels8020107
- [17] M. Suzuki, T. Takahashi, and S. Aoyagi, “Fabrication and characterization of a biodegradable hollow microneedle from chitosan,” J. Robot. Mechatron., Vol.32, No.2, pp. 401-407, 2020. https://doi.org/10.20965/jrm.2020.p0401
- [18] M. L. Fernandez, Z. Upton, H. Edwards, K. Finlayson, and G. K. Shooter, “Elevated uric acid correlates with wound severity,” Int. Wound J., Vol.9, No.2, pp. 139-149, 2012. https://doi.org/10.1111/j.1742-481X.2011.00870.x
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.