Paper:
Analysis of Cell Spheroid Morphological Characteristics Using the Spheroid Morphology Evaluation System
Takeshi Shimoto*1, Xiu-Ying Zhang*2, Shizuka Akieda*3, Atsushi Ishikawa*4, Hidehiko Higaki*4, and Koichi Nakayama*5
*1Fukuoka Institute of Technology
3-30-1 Wajiro-higashi, Higashi-ku, Fukuoka 811-0295, Japan
*2Kyushu University
3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan
*3Cyfuse Biomedical K.K.
7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
*4Kyushu Sangyo University
2-3-1 Matsukadai, Higashi-ku, Fukuoka 813-8503, Japan
*5Saga University
5-1-1 Nabeshima, Saga 849-8501, Japan
Our research group established a technology for forming three-dimensional cell constructs to regenerate osteochondro cells without scaffolding. The established technology employed spheroids to form cell constructs. We also developed a method for arranging spheroids in arbitrary positions to form cell constructs in complex shapes. However, we could only form cell constructs as expected when the formed spheroids were the appropriate sizes. This study, therefore, aimed to chronologically analyze the spheroid morphological characteristics of rabbit mechanical stem cells using the developed spheroid morphological evaluation system. We set the numbers of cells/wells as 2 × 104, 3 × 104, 4 × 104, 5 × 104, 6 × 104, and 7 × 104 and the passage number as 7. Further, we observed the cultured spheroids every 24 hours after seeding for five days. The analysis enabled us to specify an optimal range for the numbers of cells required to form spheroids with high degrees of circularity. We could also control the formed spheroid sizes by adjusting the cell count and culturing time.
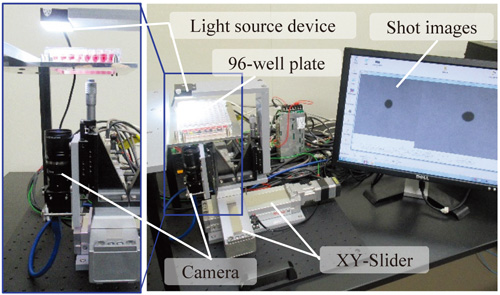
Spheroid morphological evaluation system
- [1] M. Harimoto, M. Yamato, M. Hirose, T. Takahashi, Y. Isoi, A. Kikuchi, and T. Okano, “Novel approach for achieving double-layered cell sheets co-culture: overlaying endothelial cell sheets onto monolayer hepatocytes utilizing temperature-responsive culture dishes,” J. of Biomedical Materials Research, Vol.62, No.3, pp. 464-470, 2002.
- [2] T. Matsuo, H. Masumoto, S. Tajima, T. Ikuno, S. Katayama, K. Minakata, T. Ikeda, K. Yamamizu, Y. Tabata, R. Sakata, and J. K. Yamashita, “Efficient long-term survival of cell grafts after myocardial infarction with thick viable cardiac tissue entirely from pluripotent stem cells,” Scientific Reports, Vol.5, 16842, 2015.
- [3] J. Groll, T. Boland, T. Blunk, J. A. Burdick, D. W. Cho, P. D. Dalton, B. Derby, G. Forgacs, Q. Li, V. A. Mironov, L. Moroni, M. Nakamura, W. Shu, S. Takeuchi, G. Vozzi, T. B. Woodfield, T. Xu, J. J. Yoo, and J. Malda, “Biofabrication: reappraising the definition of an evolving field,” Biofabrication, Vol.8, No.8, 013001, 2016.
- [4] A. P. Rago, D. M. Dean, and J. R. Morgan, “Controlling Cell Position in Complex Heterotypic 3D Microtissues by Tissue Fusion,” Biotechnol. Bioeng., Vol.102, No.4, pp. 1231-1241, 2009.
- [5] E. D. Miller, G. W. Fisher L. E. Weiss, L. M. Walker, and P. G. Campbell, “Dose-dependent cell growth in response to concentration modulated patterns of FGF-2 printed on fibrin,” Biomaterials, Vol.27, No.10, pp. 2213-2221, 2006.
- [6] V. L. Tsang, A. A. Chen, L. M. Cho, K. D. Jadin, R. L. Sah, S. De-Long, J. L. West, and S. N. Bhatia, “Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels,” The FASEB J., Vol.21, No.3, pp. 790-801, 2006.
- [7] M. Nakamura, A. Kobayashi, F. Takagi, A. Watanabe, Y. Hiruma, K. Ohuchi, Y. Iwasaki, M. Horie, I. Morita, and S. Takatani, “Biocompatible inkjet printing technique for designed seeding of individual living cells,” Tissue Engineering, Vol.11, Nos.11-12, pp. 1658-1666, 2005.
- [8] H. Onoe, T. Okitsu, A. Itou, M. Kato-Negishi, R. Gojo, D. Kiriya, K. Sato, S. Miura, S. Iwanaga, K. Kuribayashi-Shigetomi, Y. T. Matsunaga, Y. Shimoyama, and S. Takeuchi, “Metre-long cell-laden microfibres exhibit tissue morphologies and functions,” Nature Materials, Vol.12, No.6, pp. 584-590, 2013.
- [9] K. Kuribayashi-Shigetomi, H. Onoe, and S. Takeuchi, “Cell Origami: Self-Folding of Three-Dimensional Cell-laden microstructures driven by cell traction force,” PLoS ONE, Vol.7, No.12, e51085, 2012.
- [10] E. B. Hunziker, I. M. Driesang, and C. Saager, “Structural barrier principle for growth factor-based articular cartilage repair,” Clin. Orthop. Relat. Res., Vol.391, pp. 182-189, 2001.
- [11] P. Mainil-Varlet, F. Rieser, S. Grogan, W. Mueller, C. Saager, and R. P. Jakob, “Articular cartilage repair using a tissue-engineered cartilage-like implant: an animal study,” Osteoarthritis and Cartilage, Vol.9, pp. 6-15, 2001.
- [12] M. Ochi, N. Adachi, H. Nobuto, S. Yanada, Y. Ito, and M. Agung, “Articular cartilage repair using tissue engineering technique – Novel approach with minimally invasive procedure,” Artificial Organs, Vol.28, No.1, pp. 28-32, 2004.
- [13] T. Shimoto, K. Nakayama, S. Matsuda, and Y. Iwamoto, “Building of HD MACs using cell processing robot for cartilage regeneration,” J. Robot. Mechatron., Vol.24, No.2, pp. 347-353, 2012.
- [14] K. Ishihara, K. Nakayama, S. Akieda, S. Matsuda, and Y. Iwamoto, “Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells,” J. Orthop. Surg. Res., Vol.14, No.9, p. 98, 2014.
- [15] D. Murata, S. Tokunaga, T. Tamura, H. Kawaguchi, N. Miyoshi, M. Fujiki, K. Nakayama, and K. Misumi, “A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells,” J. Orthop. Surg. Res., Vol.18, No.10, p. 35, 2015.
- [16] H. Yurie, R. Ikeguchi, T. Aoyama, Y. Kaizawa, J. Tajino, A. Ito, S. Ohta, H. Oda, H. Takeuchi, S. Akieda, M. Tsuji, K. Nakayama, and S. Matsuda, “The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model,” PLoS ONE, Vol.12, No.2, e0171448, 2017.
- [17] A. C. Taylor, “Responses of cells to pH changes in the medium,” J. Cell Biol., Vol.15, No.2, pp. 201-209, 1962.
- [18] K. Kusamori, M. Nishikawa, Y. Takahashi, and Y. Takakura, “Development of multicellular spheroid for cell-based therapy,” Drug Delivery System, Vol.28, No.1, pp. 45-53, 2013
- [19] Y. Yanagi, K. Nakayama, T. Taguchi, S. Enosawa, T. Tamura, K. Yoshimaru, T. Matsuura, M. Hayashida, K. Kohashi, Y. Oda, T. Yamaza, and E. Kobayashi, “In vivo and ex vivo methods of growing a liver bud through tissue connection,” Sci. Rep., Vol.7, No.1, 14085, 2017.
- [20] S. Konagaya, T. Ando, T. Yamauchi, H. Suemori, and H. Iwata, “Long-term maintenance of human induced pluripotent stem cells by automated cell culture system,” Sci. Rep., Vol.5, 16647, 2015.
- [21] K. Matsuura, M. Wada, K. Konishi, M. Sato, U. Iwamoto, Y. Sato, A. Tachibana, T. Kikuchi, T. Iwamiya, T. Shimizu, J. K. Yamashita, M. Yamato, N. Hagiwara, and T. Okano, “Fabrication of mouse embryonic stem cell-derived layered cardiac cell sheets using a bioreactor culture system,” Stem. Cells. Dev., Vol.15, pp. 921-929, 2006.
 This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.
This article is published under a Creative Commons Attribution-NoDerivatives 4.0 Internationa License.